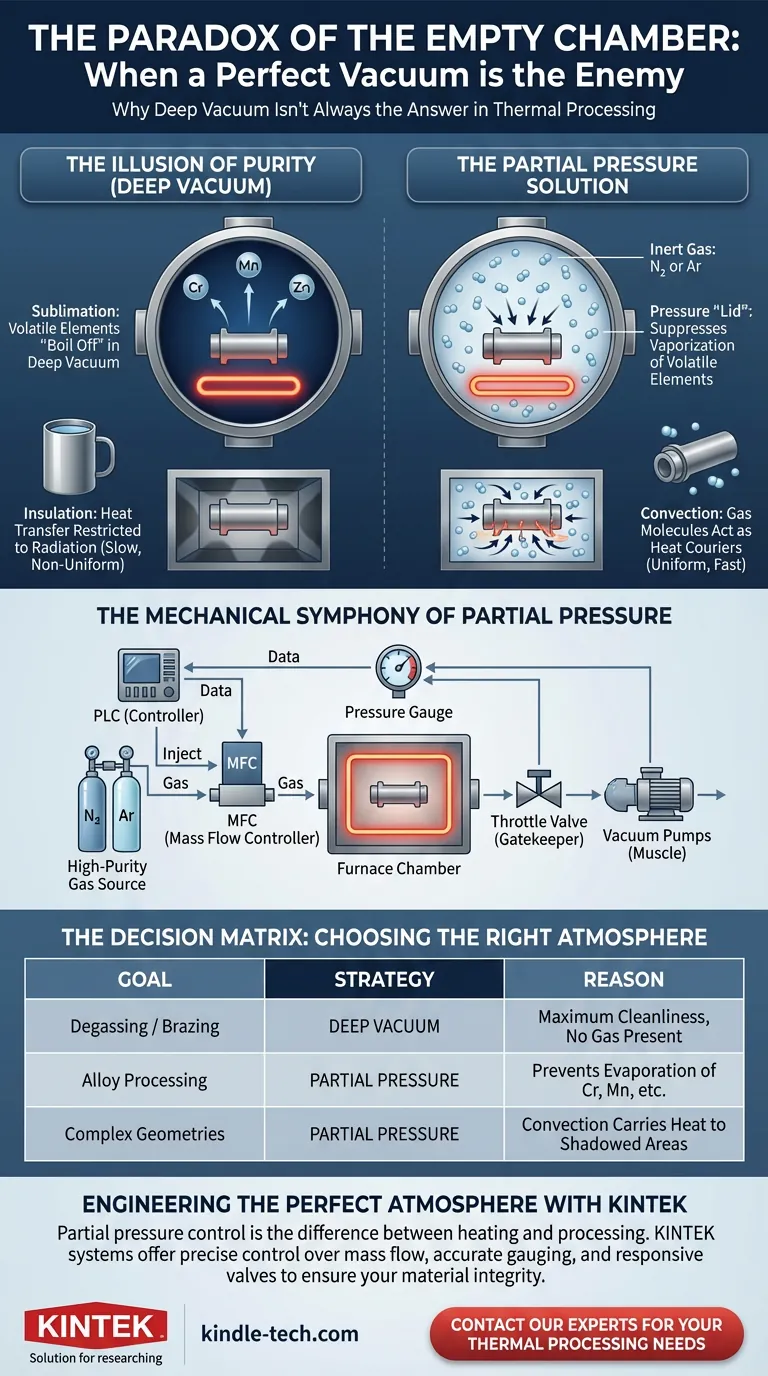
The Illusion of Purity
There is an intuitive logic to vacuum processing that feels almost philosophical: if you want a pure result, you must create a pure environment.
We assume that by removing everything—every last molecule of air, moisture, and contaminant—we protect the material inside. In the pursuit of the "perfect" vacuum, we push pumps to their limits, chasing the lowest possible pressure readings.
But in thermal processing, intuition often fails physics.
For certain high-performance alloys, a deep vacuum isn't a protective shield. It is a vacuum cleaner, violently stripping away the very elements that make the metal strong. This is where the concept of Partial Pressure enters the narrative—a technique that admits the world back in to save the process.
The Physics of Vanishing Metals
When you heat a material in a deep vacuum, you lower the boiling point of the elements contained within it.
Most engineers worry about oxidation (adding oxygen). But a more insidious threat is sublimation (losing metal). Elements with high vapor pressures do not wait to melt; under deep vacuum and high heat, they transform directly from solid to gas.
Consider the implications for common alloying elements:
- Chromium
- Manganese
- Zinc
If you process a tool steel in a deep vacuum, these elements can literally boil off the surface. You open the furnace to find a part that looks correct geometrically but has been chemically hollowed out. The surface is depleted, the hardness is compromised, and the integrity is gone.
The Partial Pressure Solution
Partial pressure acts as a "lid" on a boiling pot.
By introducing a controlled amount of inert gas (usually Nitrogen or Argon) back into the chamber, you create a physical barrier. This artificially induces pressure—typically between 10 to 1000 mbar—which suppresses the vaporization of volatile elements.
It preserves the chemical recipe of your alloy.
The Silent Problem of Heat Transfer
There is a second, often overlooked reason to abandon the deep vacuum: Insulation.
A vacuum is an exceptional thermal insulator. That is why high-end travel mugs work. But in a furnace, insulation is the enemy. In a deep vacuum, heat can only move via radiation. This creates two problems:
- Line-of-Sight Limitations: Radiation only heats what it "sees." Shadowed areas of complex geometries remain cool.
- Slow Equilibrium: Waiting for radiant heat to equalize across a dense load takes time.
Convection as a Catalyst
When you introduce a partial pressure gas, you reintroduce convection.
Gas molecules act as couriers. They pick up heat from the heating elements and physically carry it into the deep recesses of the workload. This results in:
- Tighter temperature uniformity.
- Reduced cycle times.
- Consistent outcomes for complex, dense loads.
The Mechanical Symphony
Implementing partial pressure transforms a furnace from a static evacuation chamber into a dynamic flow system. It is a sophisticated balancing act managed by a Programmable Logic Controller (PLC).
The system must maintain equilibrium through three key components:
- The Conductor (MFC): A Mass Flow Controller precisely meters high-purity gas into the chamber.
- The Muscle (Vacuum Pumps): The pumps do not stop; they continue to pull, ensuring flow direction.
- The Gatekeeper (Throttle Valve): An adjustable valve on the outlet dynamically opens or closes to restrict the exit speed.
The PLC watches the pressure gauge. If the pressure drops too low, it constricts the valve or adds more gas. If it spikes, it opens the valve. This loop happens continuously, maintaining a precise atmosphere that is neither a vacuum nor standard air.
The Risk of Complexity
The shift from static vacuum to partial pressure is a shift from brute force to finesse. It introduces variables that must be respected.
- Purity is Paramount: If your "inert" gas contains moisture or oxygen, you are effectively injecting contaminants directly into the hot zone.
- Pump Stress: Continuous gas flow changes the load profile on vacuum pumps, requiring different maintenance protocols.
It requires a shift in mindset: you are no longer just removing air; you are building an atmosphere.
Summary: The Decision Matrix
Not every process requires partial pressure. But for those that do, it is non-negotiable.
| Goal | Strategy | Reason |
|---|---|---|
| Degassing / Brazing | Deep Vacuum | Maximum cleanliness is required; no gas should be present. |
| Alloy Processing | Partial Pressure | Prevents the evaporation of Chromium, Manganese, etc. |
| Complex Geometries | Partial Pressure | Gas molecules carry heat to shadowed areas (Convection). |
Engineering the Perfect Atmosphere
Partial pressure control is the difference between a furnace that merely heats and one that processes. It requires equipment that offers not just power, but precision—tight control over mass flow, accurate gauging, and responsive valves.
At KINTEK, we understand that modern laboratory needs rarely fit into a "one size fits all" category. Our vacuum furnace systems are designed to handle the nuance of partial pressure, ensuring that your manganese stays in your alloy and your heat reaches every corner of your workload.
Don't let the physics of vacuums work against your materials.
Contact Our Experts to discuss your specific thermal processing needs and find a solution that offers the perfect balance of pressure and purity.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
Related Articles
- Hydrogen Atmosphere Furnaces: Applications, Safety, and Maintenance
- Comprehensive Guide to Atmosphere Furnaces: Types, Applications, and Benefits
- The Silent Saboteur in Your Furnace: Why Your Heat Treatment Fails and How to Fix It
- Muffle Furnace: Unraveling the Secrets of Uniform Heating and Controlled Atmosphere
- Exploring the Using a Chamber Furnace for Industrial and Laboratory Applications




















