It is easy to romanticize the chemical reaction. We focus on the sample, the temperature curve, and the data produced. We rarely pause to consider the vessel itself until it fails.
In high-temperature thermodynamics, the tube furnace tube is not merely a container. It is the boundary condition. It is the only thing standing between a controlled experiment and a catastrophic breach.
When experiments fail, they often do so not because the chemistry was wrong, but because the engineer ignored the fundamental nature of the material holding it. They prioritized heat over shock resistance, or visibility over porosity.
The choice of a furnace tube is not a hunt for the "best" material. In engineering, "best" does not exist. There are only trade-offs.
The Irony of Perfection
We want a material that can withstand 2000°C, is perfectly transparent, allows for a high vacuum, and can be heated instantly without cracking. Physics dictates that this material does not exist.
To select the right tube, you must accept a compromise. You are balancing three competing forces:
- Thermal Ceiling: How hot can it get?
- Thermal Shock: How fast can it change?
- Atmospheric Integrity: How well does it seal?
Your decision requires a deep understanding of your process's specific intolerance. What are you willing to sacrifice?
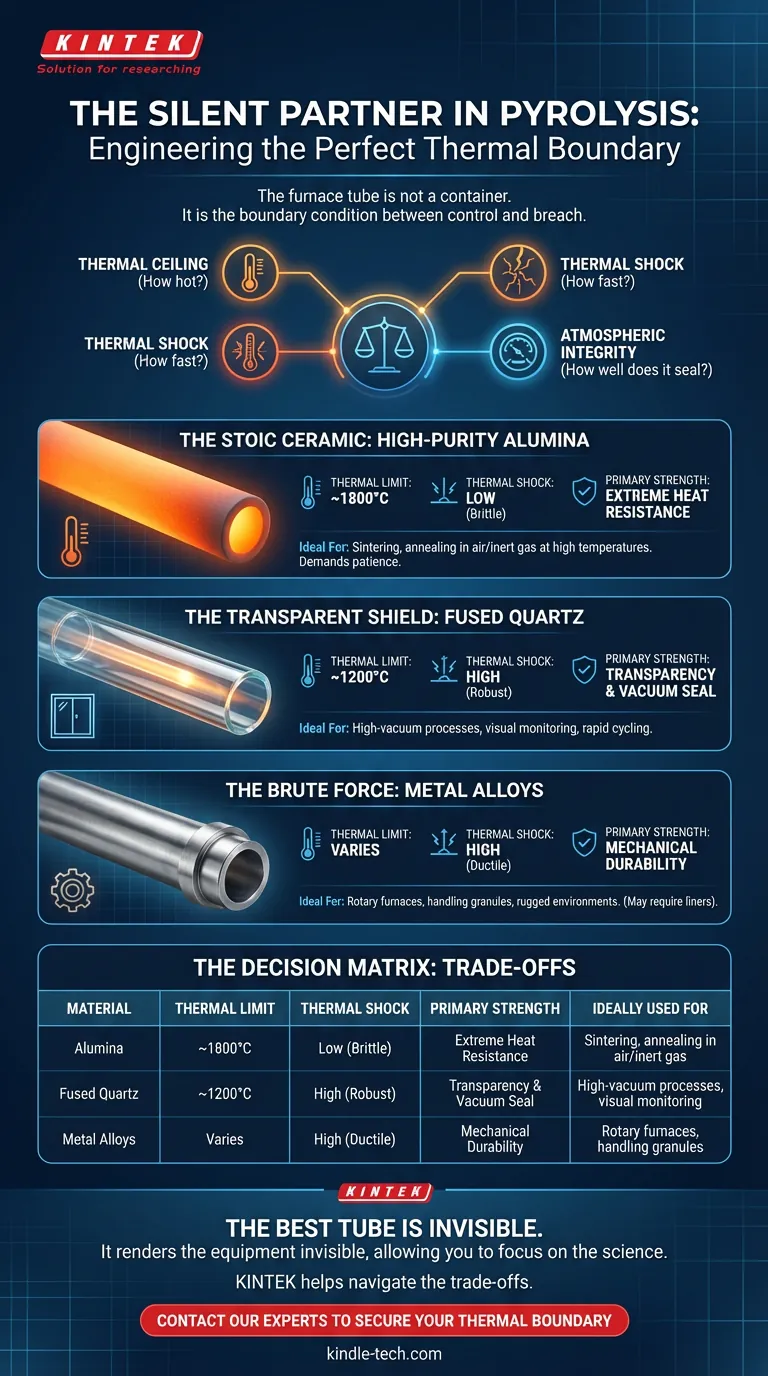
The Stoic Ceramic: High-Purity Alumina
Alumina is the workhorse of the high-temperature world. It is the choice when raw heat is the primary variable.
Dense and chemically inert, high-purity alumina can withstand punishing temperatures up to 1700°C or even 1800°C. It stands firm in air and inert atmospheres where other materials would soften or vaporize.
The Trade-off: Alumina has a rigid personality. It is susceptible to thermal shock.
If you heat it too fast or cool it too aggressively, the thermal gradients create stress fractures, and the tube will crack. It demands patience. It is also slightly porous at the microscopic level, making it challenging (though not impossible) to use in ultra-high vacuum applications without specialized glazing.
The Transparent Shield: Fused Quartz
There is a psychological comfort in seeing your experiment. Fused Quartz offers this. It is a high-purity glass that provides a window into the reaction.
Its engineering superpower, however, is not just transparency. It is thermal shock resistance. You can subject quartz to rapid temperature swings that would shatter alumina, and it will remain intact.
Because it is non-porous, quartz is also the gold standard for high-vacuum applications. It provides a seal integrity that ceramics struggle to match.
The Trade-off: It has a lower thermal ceiling. Typically capped at 1100°C to 1200°C, quartz will begin to devitrify or soften if pushed beyond its limits. It is a shield for delicate, visible, vacuum-sealed processes, not for extreme heat.
The Brute Force: Metal Alloys
Sometimes, you need mechanical ductility. Stainless Steel and superalloys like Inconel bring physical toughness to the laboratory.
These tubes do not shatter. They are ideal for rougher applications, such as rotary tube furnaces processing heavy powders or granules. They handle the physical abrasion of tumbling materials far better than glass or ceramic ever could.
The Trade-off: Metal reacts. At high temperatures, metal tubes can outgas or react with the sample.
To mitigate this, engineers often have to design complex workarounds, such as inserting non-metallic inner liners to prevent the volatile components of a sample from touching the alloy walls.
The Decision Matrix
Making the right choice is about aligning the material’s physics with your experiment’s constraints.
Here is the breakdown of the trade-offs:
| Material | Thermal Limit | Thermal Shock | Primary Strength | Ideally Used For |
|---|---|---|---|---|
| Alumina | ~1800°C | Low (Brittle) | Extreme Heat Resistance | Sintering, annealing in air/inert gas at high temps. |
| Fused Quartz | ~1200°C | High (Robust) | Transparency & Vacuum Seal | High-vacuum processes, visual monitoring, rapid cycling. |
| Metal Alloys | Varies | High (Ductile) | Mechanical Durability | Rotary furnaces, handling granules, rugged environments. |
The Cost of Uncertainty
In the lab, uncertainty is expensive. A cracked tube results in lost samples, damaged heating elements, and downtime.
The "best" tube is simply the one that renders the equipment invisible. It performs its function so well that you forget it is there, allowing you to focus entirely on the science.
At KINTEK, we understand that you aren't just buying a tube; you are buying the assurance of a controlled environment. We specialize in high-purity lab equipment, helping you navigate the trade-offs between thermal limits and mechanical needs.
Whether you require the extreme heat resistance of alumina or the vacuum integrity of quartz, our role is to ensure your equipment never becomes the variable that ruins the experiment.
Contact Our Experts to analyze your process parameters and secure the exact thermal boundary your research demands.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
Related Articles
- Why Your Furnace Components Keep Failing—And the Material Science Fix
- Muffle vs. Tube Furnace: How the Right Choice Prevents Catastrophic Lab Failure
- Your Tube Furnace Is Not the Problem—Your Choice of It Is
- Why Your Ceramic Furnace Tubes Keep Cracking—And How to Choose the Right One
- Entropy and the Alumina Tube: The Art of Precision Maintenance




















