Imagine this: hours into a critical loss-on-ignition test, you open the furnace door expecting a fine, white ash. Instead, you're met with a puff of acrid smoke and a disappointing sight: a blackened, half-burned chunk of material. The sample is ruined, your timeline is shot, and you're left wondering, "I set the temperature correctly. What went wrong?"
If this scenario feels painfully familiar, you are not alone.
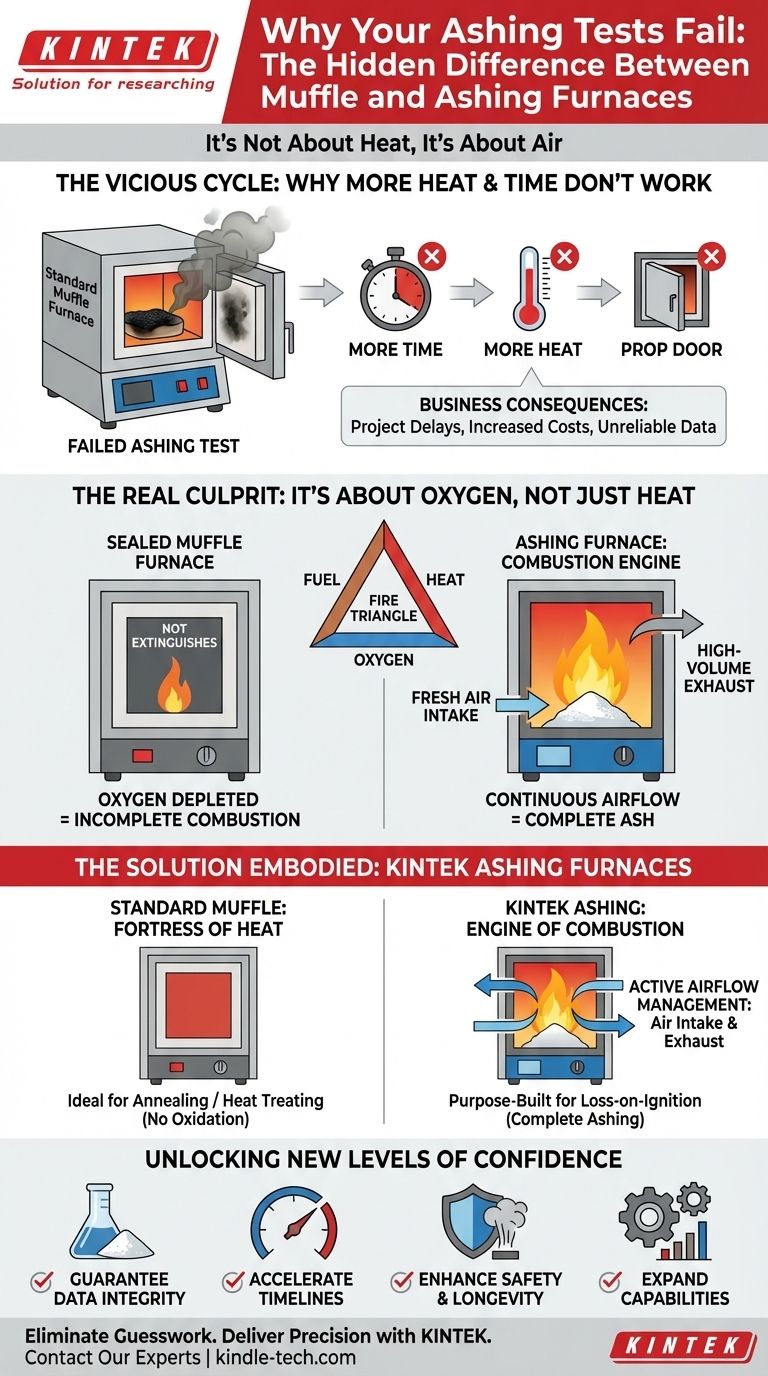
The Vicious Cycle: Why More Heat and Time Don't Work
This frustrating failure is a common story in labs worldwide. When faced with incomplete combustion, the typical response is a series of logical but ultimately futile adjustments.
First, you might try running the cycle for longer. When that fails to produce a clean ash, you might increase the setpoint temperature, assuming more heat is the answer. Some may even resort to the risky practice of propping the furnace door open slightly, hoping to "help it along."
Each attempt leads to the same result: inconsistent data, wasted samples, and hours of lost productivity. The business consequences are significant:
- Project Delays: Critical quality control checks become a bottleneck, holding up production or research.
- Increased Costs: Wasted materials, high energy consumption from repeated tests, and—most damagingly—the potential for corrosive fumes to degrade the furnace's expensive heating elements and insulation.
- Unreliable Data: Inaccurate ash content analysis can compromise product quality, lead to failed audits, and damage your organization's reputation for precision.
These "fixes" all fail for the same reason: they are treating the wrong symptom. The problem isn't your temperature or your timing.
The Real Culprit: It's Not About Heat, It's About Air
The fundamental reason for incomplete combustion is surprisingly simple: you're trying to light a fire in a sealed box.
Every fire, from a campfire to a complex laboratory combustion, needs three things: fuel (your sample), heat (your furnace), and oxygen. A standard muffle furnace is engineered to excel at providing heat in a stable, static environment. Its very design—the "muffle"—is meant to isolate the sample from the outside world, which means it also severely limits the supply of fresh air.
Within minutes of the cycle starting, the combustion process consumes the small amount of oxygen inside the chamber and then stops. No amount of extra heat or time can restart it. You're left with a baked, charred sample, not a fully combusted ash. This is why common "fixes" fail:
- More Time: Is useless when the chemical reaction has already been starved of oxygen.
- More Heat: Only bakes the carbonized material further without actually burning it.
- Propping the Door: Is an uncontrolled, unsafe method that introduces unpredictable variables and fails to vent hazardous fumes effectively.
To achieve complete and rapid combustion, you don't need a hotter furnace. You need a furnace that is built to breathe.
The Solution Embodied: A Tool Designed for Combustion
The solution is not to find a workaround, but to use the correct tool designed from the ground up to solve the oxygen problem. This is the core principle behind a true ashing furnace.
An ashing furnace is not just a muffle furnace with a different name; it is an engine of combustion. While it shares the same indirect heating principle, it adds a critical system that standard muffle furnaces lack: active airflow management.
Our KINTEK ashing furnaces are the embodiment of this principle. They are purposefully engineered with:
- Continuous Air Intake: A dedicated intake port constantly supplies the chamber with fresh, oxygen-rich air, often pre-heated to maintain thermal uniformity and prevent shocking the sample.
- High-Volume Exhaust: A large, dedicated vent actively and safely removes the smoke, moisture, and corrosive fumes generated during combustion, protecting both the lab environment and the furnace components.
This engineered airflow directly addresses the root cause of failure. It ensures the sample has a constant, abundant supply of oxygen, allowing the combustion process to proceed to completion quickly and efficiently. It’s a tool built from a deep understanding of the underlying chemistry, designed to give you a perfect result every time.
Conversely, our standard KINTEK muffle furnaces are designed for the opposite goal: creating a fortress of heat. They provide a pristine, static atmosphere, perfect for applications like annealing or heat-treating metals where introducing oxygen would cause unwanted oxidation and ruin the sample.
Beyond the Fix: Unlocking New Levels of Confidence and Efficiency
When you stop fighting your equipment and start using the right tool for the job, everything changes. The nagging uncertainty of thermal processing is replaced by predictable success. This doesn't just solve an old problem; it unlocks new potential for your entire lab.
With reliable, repeatable ashing, you can now:
- Guarantee Data Integrity: Confidently perform loss-on-ignition and ash content analysis that meets the most stringent industry standards (like ASTM or ISO), knowing your results are accurate.
- Accelerate Timelines: Eliminate wasted hours on re-runs. Complete tests correctly the first time, freeing up valuable personnel and equipment to focus on more innovative work.
- Enhance Safety & Longevity: Operate in a safer lab environment, free from hazardous fumes, while protecting your furnace from the corrosive byproducts that shorten its lifespan.
- Expand Capabilities: Take on more demanding projects, tighten quality control on your production line, and speed up the R&D of new materials, all built on a foundation of trustworthy analytical data.
Moving from inconsistent results to guaranteed accuracy isn't about working harder; it's about understanding the core problem and choosing the purpose-built solution. Let us help you eliminate the guesswork so you can focus on the results. If your projects demand precision, our team can ensure you have the right tool to deliver it.
Ready to put an end to failed tests and unreliable data? Let's discuss the specific demands of your application. Contact Our Experts.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
Related Articles
- Muffle vs. Tube Furnace: How the Right Choice Prevents Catastrophic Lab Failure
- Box Furnace vs. Muffle Furnace: Are You Using the Wrong Tool for the Job?
- Comprehensive Guide to Muffle Furnaces: Applications, Types, and Maintenance
- Why Your High-Temperature Experiments Fail: The Furnace Flaw Most Labs Overlook
- Why Your High-Temperature Experiments Fail: It's Not the Heat, It's the Furnace




















