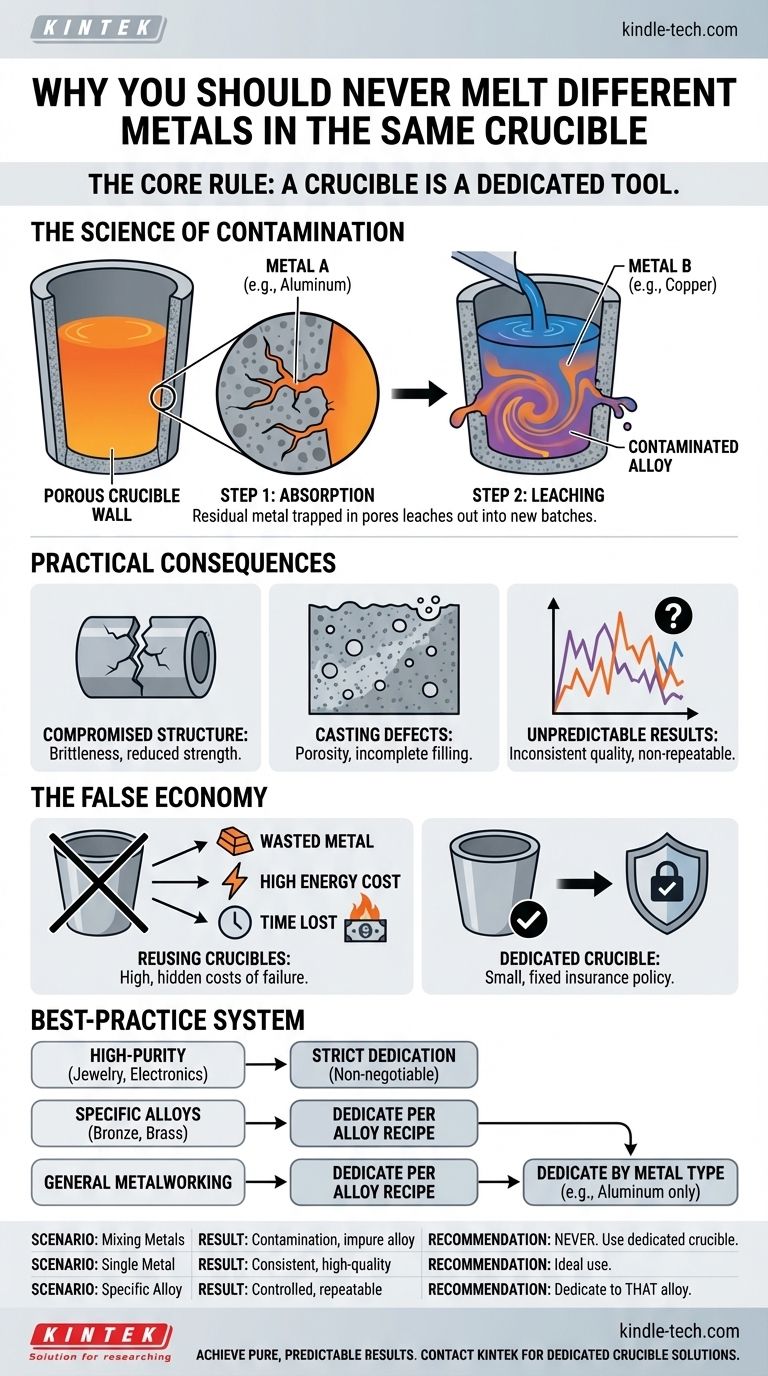
As a rule, you should never melt different metals in the same crucible. While a crucible may be rated to handle the temperatures for various metals like aluminum, brass, or iron, using it for more than one type will cause contamination. Residual metal from the first melt will inevitably mix with the second, creating an unintended and impure alloy that leads to poor quality and unpredictable castings.
The core principle is this: A crucible is a dedicated tool for a single metal or a specific, consistent alloy. Treating it otherwise introduces variables that undermine the quality, integrity, and predictability of your metalwork.

The Science of Crucible Contamination
To understand why this rule is so critical, we need to look at the material science of the crucible itself and how it interacts with molten metal.
How Crucibles Absorb Metal
Most common crucibles, such as those made from clay-graphite or silicon carbide, are inherently porous on a microscopic level.
When metal is in its liquid state, it has the ability to seep into these tiny pores and crevices within the crucible's inner wall. This process is unavoidable.
The Problem of "Leaching"
After a melt is poured, a small but significant amount of that metal remains trapped within the crucible's porous surface.
When you introduce a new, different type of metal and bring it to melting temperature, the residual metal from the previous melt will "leach" out of the walls and mix with the new batch.
Creating Unintended and Impure Alloys
This leaching directly contaminates your new melt. For example, if you melt aluminum and then attempt to melt copper in the same crucible, the residual aluminum will contaminate the copper.
You will not be casting pure copper; you will be casting an uncontrolled aluminum-bronze alloy. This new, unintended alloy will not have the properties you expect from the pure metal.
The Practical Consequences of Cross-Contamination
This contamination isn't just a theoretical problem; it has severe, tangible effects on the final product.
Compromised Structural Integrity
Even tiny amounts of a foreign metal can disrupt the crystalline structure that forms as the primary metal cools and solidifies.
This disruption can create weak points, leading to brittleness, reduced tensile strength, or poor ductility in the final cast object.
Altered Casting Properties
Contamination changes the fundamental properties of the melt itself. It can alter the ideal melting point, viscosity, and flow characteristics.
This often results in common casting defects, such as incomplete mold filling, porosity (gas bubbles trapped in the metal), or a poor surface finish.
Inconsistent and Unpredictable Results
For any professional or serious hobbyist, repeatability is key. Cross-contamination makes this impossible.
Because the amount of leached metal is unknown and inconsistent, every casting produced from a contaminated crucible will be a gamble with unpredictable results.
Understanding the Trade-offs
The primary motivation for reusing a crucible is typically to save money or space. However, this is a false economy that overlooks the true costs of contamination.
The High Cost of a Failed Casting
A new, dedicated crucible has a fixed cost. In contrast, the cost of a single failed casting is multifaceted and often much higher.
Consider the wasted metal, the significant energy consumed by the furnace, the time spent on the entire process, and the potential for a damaged mold. The cost of a dedicated crucible is a small insurance policy against these much larger losses.
The Exception: Dedicated Alloy Crucibles
The only time different metals are mixed is for the intentional creation of an alloy like bronze (copper and tin) or brass (copper and zinc).
Even in this scenario, best practice dictates using a crucible dedicated only to that specific alloy. You would not use your bronze crucible to melt pure aluminum, as this would contaminate future batches of bronze.
Implementing a Best-Practice System
Adopting a strict system for your crucibles is the most effective way to guarantee the quality of your work. The best approach depends on your specific goals.
- If your primary focus is high-purity casting (e.g., jewelry, electronics): Strict crucible dedication is non-negotiable, as even trace contamination can completely ruin the electrical, thermal, or aesthetic properties of the final product.
- If your primary focus is creating specific alloys (e.g., bronze, brass): Use a dedicated crucible for each specific alloy recipe to maintain precise, repeatable ratios and ensure consistent results for every batch.
- If your primary focus is general metalworking or hobby casting: The most effective way to improve your results is to dedicate crucibles by metal type (e.g., one clearly marked for aluminum, one for brass).
Dedicating your crucibles is a foundational discipline that separates inconsistent results from professional-grade metalwork.
Summary Table:
| Crucible Use Scenario | Result | Recommendation |
|---|---|---|
| Melting different metals sequentially | Cross-contamination, impure alloys, failed castings | Never do this. Use a dedicated crucible for each metal type. |
| Melting a single, pure metal | Consistent, high-quality results with predictable properties | Ideal use case for a dedicated crucible. |
| Creating a specific, intentional alloy (e.g., bronze) | Controlled, repeatable alloy with consistent properties | Use a crucible dedicated only to that specific alloy recipe. |
Achieve pure, predictable, and professional results with every melt.
Don't let crucible contamination waste your valuable time, materials, and effort. KINTEK specializes in providing the right lab equipment, including high-quality, dedicated crucibles for every metal and alloy, to ensure the integrity of your work.
Contact our experts today to find the perfect crucible solution for your specific metals and casting needs, and start producing consistent, high-quality results.
Visual Guide
Related Products
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- What are 2 uses of crucible? Mastering High-Temperature Melting and Analysis
- What is a crucible material for a furnace? A Guide to Choosing the Right High-Temperature Container
- How do you clean a melting crucible? Protect Your Crucible and Ensure Metal Purity
- What is the best type of crucible? The Answer Depends on Your Application's Needs
- Why is a PTFE crucible preferred for plasma etching? Ensure Chemical Integrity and Targeted Action



















