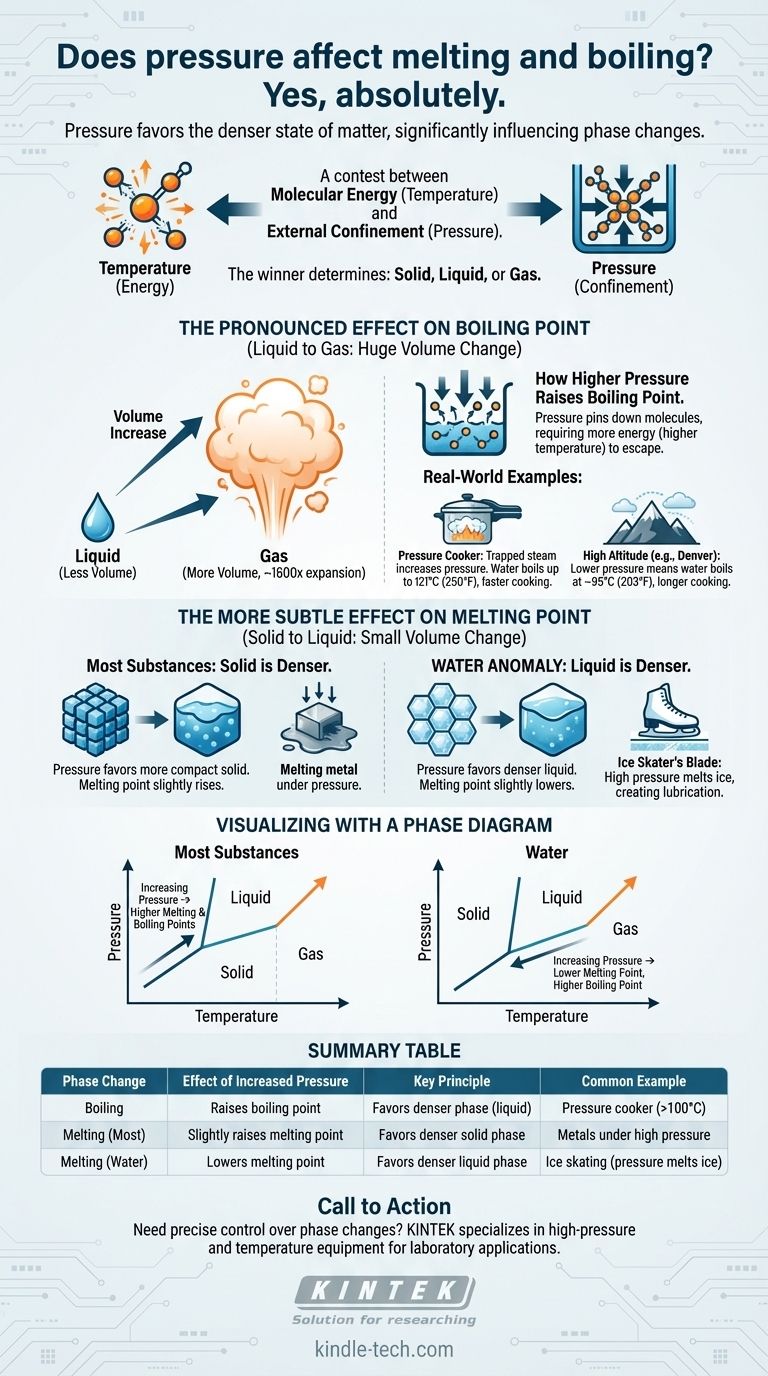
Yes, absolutely. Pressure is a critical factor that directly influences the temperatures at which substances change phase. Increasing external pressure generally raises the boiling point of a liquid significantly. Its effect on the melting point is more subtle but follows a clear principle: pressure favors the denser state of matter.
The core principle is a contest between molecular energy (temperature) and external confinement (pressure). Temperature gives molecules the energy to break free into a less orderly state, while pressure forces them together into a more compact, denser state. The winner of this contest determines if a substance is solid, liquid, or gas.

Why Pressure Influences Phase Changes
The Battle Between Energy and Confinement
A phase change, like melting or boiling, occurs when molecules gain enough thermal energy to overcome the forces holding them in a fixed structure. Temperature provides this energy.
Pressure, on the other hand, is an external force pushing the molecules together. It acts as a form of confinement, making it more difficult for molecules to spread out and transition into a less dense phase.
A Matter of Volume and Density
The key to understanding pressure's effect lies in volume. When a substance melts or boils, its volume and density change.
Pressure will always favor the phase that takes up less volume—the denser phase. This single rule explains why pressure affects boiling and melting differently.
The Pronounced Effect on Boiling Point
From Liquid to Gas: A Huge Volume Change
The transition from a liquid to a gas involves a massive increase in volume. A single drop of water, for example, expands to fill a space over 1,600 times larger when it becomes steam.
Because this volume change is so large, pressure has a very strong and direct impact on the boiling point.
How Higher Pressure Raises the Boiling Point
When you increase the pressure on a liquid's surface, you are essentially "pinning down" the molecules. They now require significantly more kinetic energy (a higher temperature) to escape into the gas phase.
This is why the boiling point of nearly all substances increases as pressure increases.
Real-World Example: The Pressure Cooker
A pressure cooker works by sealing a pot, trapping the steam produced from boiling water. This trapped steam dramatically increases the pressure inside.
Under this high pressure, the boiling point of water can rise from 100°C (212°F) to as high as 121°C (250°F). This hotter water transfers heat more quickly, drastically reducing cooking times.
Real-World Example: Cooking at High Altitude
The opposite occurs at high altitudes. In Denver, Colorado (at 5,280 feet), the atmospheric pressure is lower.
With less atmospheric pressure holding the water molecules down, they can escape into the gas phase more easily. Water there boils at around 95°C (203°F), which means food must be cooked for a longer time.
The More Subtle Effect on Melting Point
From Solid to Liquid: A Small Volume Change
In contrast to boiling, the volume change during melting is very small. The densities of a substance in its solid and liquid forms are usually quite similar.
Because the volume change is minimal, pressure has a much less dramatic effect on the melting point.
For Most Substances: Higher Pressure Raises Melting Point
Most materials—from metals to waxes to carbon dioxide—are denser in their solid form than their liquid form.
In these cases, increasing pressure favors the more compact solid phase. This makes it slightly harder to melt, so a higher temperature is required. The melting point rises with pressure.
The Anomaly of Water: A Crucial Exception
Water is a remarkable and rare exception. Solid water (ice) is less dense than liquid water, which is why ice floats.
Because liquid water is the denser phase, increasing pressure on ice favors the formation of liquid. This means that under high pressure, ice will melt at a temperature below 0°C (32°F).
Real-World Example: The Ice Skater's Blade
The classic illustration of this principle is an ice skater. The thin blade of the skate concentrates the skater's entire weight onto a tiny area, creating immense pressure on the ice.
This high pressure causes the ice directly beneath the blade to melt at a slightly lower temperature, creating a microscopic layer of water that lubricates the blade's path.
Understanding the Trade-offs: Visualizing with a Phase Diagram
A phase diagram is a simple map that shows the state of a substance (solid, liquid, or gas) at any combination of temperature and pressure.
The Liquid-Gas Boundary
The line separating the liquid and gas phases always slopes up and to the right. This visually confirms that as you increase pressure (moving up the vertical axis), you must also increase the temperature (moving right on the horizontal axis) to make the substance boil.
The Solid-Liquid Boundary
The line between the solid and liquid phases is nearly vertical, showing that pressure has a much smaller influence on melting.
For most substances, this line tilts slightly to the right (higher pressure, higher melting point). For water, this line uniquely tilts to the left, showing that higher pressure leads to a lower melting point.
Key Principles for Practical Application
To apply this knowledge effectively, focus on the substance and the type of phase change involved.
- If you are dealing with boiling or condensation: Remember that pressure is the dominant factor. Higher pressure means a higher boiling point, and lower pressure means a lower boiling point.
- If you are dealing with melting or freezing (for most materials): The effect of pressure is minor. Higher pressure will slightly increase the melting point by favoring the denser solid state.
- If you are dealing specifically with water ice: Remember it is the exception. Higher pressure lowers the freezing/melting point by favoring the denser liquid state.
Understanding how pressure and temperature interact gives you direct control over the physical state of matter.
Summary Table:
| Phase Change | Effect of Increased Pressure | Key Principle | Common Example |
|---|---|---|---|
| Boiling | Raises boiling point | Favors denser phase (liquid) | Pressure cooker (water boils >100°C) |
| Melting (Most Materials) | Slightly raises melting point | Favors denser solid phase | Metals under high pressure |
| Melting (Water/Ice) | Lowers melting point | Favors denser liquid phase | Ice skating (pressure melts ice) |
Need precise control over phase changes in your lab processes? KINTEK specializes in high-pressure and temperature equipment for laboratory applications. Whether you're developing new materials, conducting chemical synthesis, or studying phase behavior, our reactors and ovens provide the exact pressure and temperature control you need. Contact our experts today to discuss how our solutions can enhance your research efficiency and accuracy.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What are the methods of synthesis of nanomaterials? Top-Down vs. Bottom-Up Approaches Explained
- What is magnetron sputtering coating? A High-Performance Thin Film Deposition Process
- What is preventive maintenance in a laboratory? A Proactive Strategy for Lab Reliability and Data Integrity
- What are the industrial applications of melting? A Guide to Material Control in Manufacturing
- Why is it necessary to control the temperature during composite specimen preparation? Ensure Flawless Resin Infusion
- What are the advantages of microwave heating for HEA catalysts? Unlock Superior OER Efficiency with Rapid Sintering
- What are the properties of isotropic graphite? A Guide to Its Uniform Strength & Thermal Performance
- What temperature does KBr dry at for IR? The Key to Moisture-Free, High-Quality Spectra



















