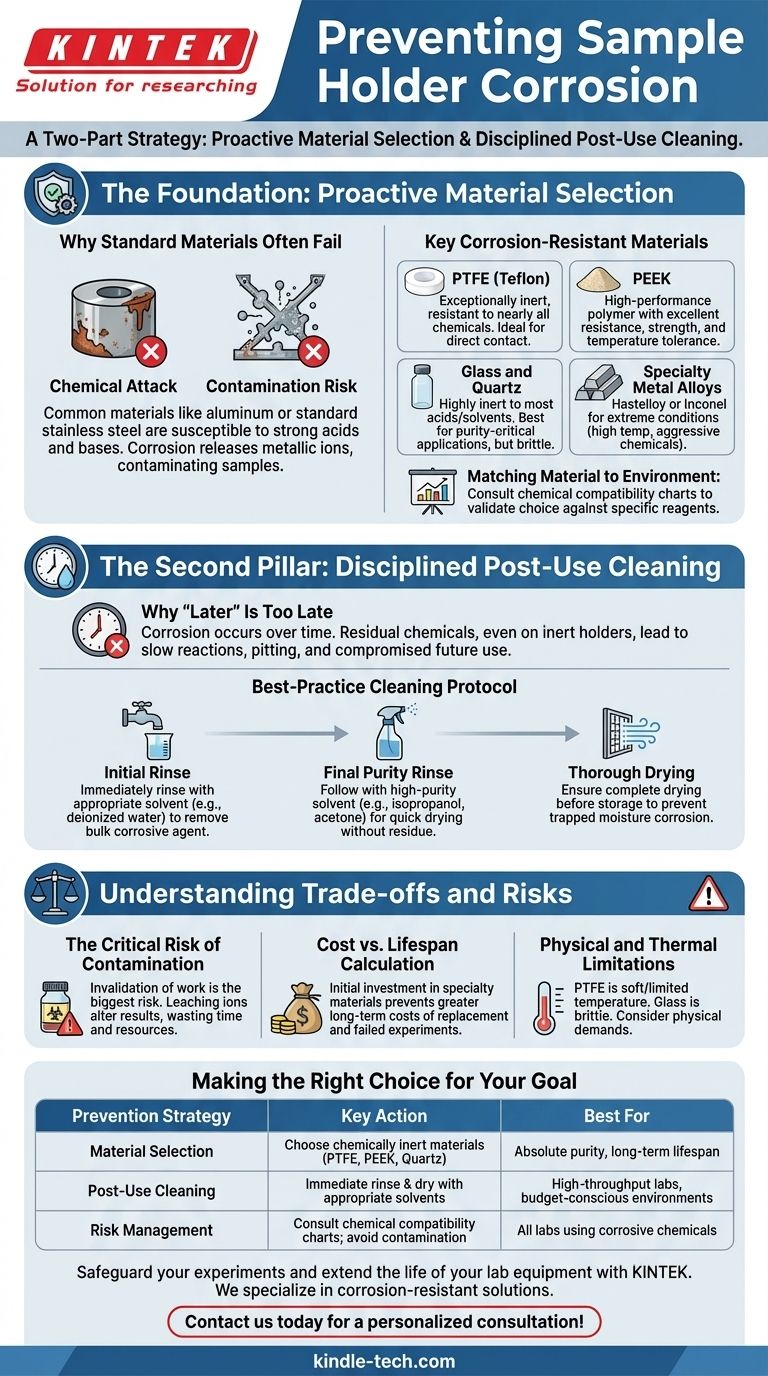
To prevent sample holder corrosion, you must employ a two-part strategy. First, select a sample holder constructed from a material that is chemically resistant to the specific corrosive reagents you are using. Second, it is critical to thoroughly clean the holder immediately after use to remove any residual chemicals that could cause damage over time.
The core challenge isn't just protecting a piece of equipment from visible rust. The deeper goal is to guarantee the integrity of your work by preventing chemical contamination and ensuring the longevity of your tools through proactive material selection and disciplined operational procedures.

The Foundation: Proactive Material Selection
The most effective way to combat corrosion is to choose a material that is fundamentally inert to your chemical environment. This decision prevents the corrosive reaction from ever beginning.
Why Standard Materials Often Fail
Common materials like aluminum or standard stainless steel are susceptible to attack by strong acids, bases, and even certain salts. This chemical reaction, or corrosion, can dissolve the holder's material, releasing metallic ions that contaminate your sample.
Key Corrosion-Resistant Materials
Your choice of material must be matched to the specific chemical agent.
- PTFE (Teflon): Exceptionally inert and resistant to nearly all chemicals. It is a common choice for holders that come into direct contact with a wide range of corrosive samples.
- PEEK (Polyether ether ketone): A high-performance polymer that offers excellent chemical resistance combined with superior strength and temperature tolerance compared to PTFE.
- Glass and Quartz: Highly inert to most acids and solvents, making them ideal for purity-critical applications. Their primary drawback is brittleness.
- Specialty Metal Alloys: For extreme conditions involving high temperatures and aggressive chemicals, alloys like Hastelloy or Inconel offer a robust solution where polymers may fail.
Matching Material to Environment
There is no single "best" material for all situations. A material resistant to a strong acid may not be suitable for a strong base or a specific organic solvent. Always consult a chemical compatibility chart to validate your material choice against the reagents in use.
The Second Pillar: Disciplined Post-Use Cleaning
Even corrosion-resistant materials can degrade with prolonged exposure. A strict and immediate cleaning protocol is a non-negotiable part of a robust corrosion prevention strategy.
Why "Later" Is Too Late
Corrosion is a process that occurs over time. Leaving residual chemicals on a holder, even an inert one, allows for slow-moving reactions, especially at connection points or in microscopic surface imperfections. This can lead to pitting, staining, or absorption that compromises future use.
A Best-Practice Cleaning Protocol
A simple rinse is often insufficient.
- Initial Rinse: Immediately after use, rinse the holder with an appropriate solvent, such as deionized water for most acids and bases, to remove the bulk of the corrosive agent.
- Final Purity Rinse: Follow up with a rinse using a high-purity solvent like isopropanol or acetone. This displaces the initial rinse water and helps the holder dry quickly without residue.
- Thorough Drying: Ensure the holder is completely dry before storage. Trapped moisture can create its own corrosive environment over time.
Understanding the Trade-offs and Risks
An effective strategy requires balancing cost, performance, and the potential consequences of failure.
The Critical Risk of Contamination
The most significant risk of a corroding holder is not the cost of replacement, but the invalidation of your work. A holder that leaches unwanted ions into your sample can subtly or completely alter your results, wasting time and resources.
The Cost vs. Lifespan Calculation
Specialty materials like PEEK or PTFE are more expensive upfront than standard metals. However, this initial investment prevents the far greater long-term costs associated with frequent equipment replacement and, more importantly, failed or repeated experiments.
Physical and Thermal Limitations
Resistant materials have their own trade-offs. PTFE is soft and has a limited temperature range. Glass is brittle and cannot withstand mechanical shock. Your choice must account for the physical and thermal demands of your process, not just the chemical ones.
Making the Right Choice for Your Goal
Select your strategy by identifying your most critical priority.
- If your primary focus is absolute experimental purity: Prioritize the most inert materials available, such as quartz or PTFE, and accept their potential physical limitations.
- If your primary focus is durability in a high-throughput environment: Choose robust materials like PEEK or specialty alloys and enforce a mandatory, immediate post-use cleaning protocol.
- If your primary focus is managing a tight budget: Recognize that using lower-cost, less-resistant materials necessitates the most rigorous cleaning discipline and carries the highest risk of sample contamination.
By integrating proactive material selection with disciplined cleaning procedures, you safeguard the integrity of your equipment and the validity of your results.
Summary Table:
| Prevention Strategy | Key Action | Best For |
|---|---|---|
| Material Selection | Choose chemically inert materials (e.g., PTFE, PEEK, Quartz) | Absolute purity, long-term equipment lifespan |
| Post-Use Cleaning | Immediate rinse & dry with appropriate solvents | High-throughput labs, budget-conscious environments |
| Risk Management | Consult chemical compatibility charts; avoid contamination | All labs using corrosive chemicals |
Safeguard your experiments and extend the life of your lab equipment. Corrosion can lead to costly sample contamination and invalidated results. At KINTEK, we specialize in providing corrosion-resistant lab equipment and consumables—including PTFE, PEEK, and quartz sample holders—perfect for laboratories handling aggressive chemicals. Let our experts help you select the right materials to protect your work. Contact us today for a personalized consultation!
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Customizable PTFE Wafer Carriers for Semiconductor and Lab Applications
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Special Heat Press Mold for Lab Use
People Also Ask
- What is the minimum sample required for XRD analysis? Optimize Your Material Analysis
- Why is an airtight sample holder with a beryllium window required for XRD of sulfide solid electrolytes?
- What is the difference between XRF and XRD techniques? A Guide to Choosing the Right Analytical Tool
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference
- How should a sample holder be handled to ensure its longevity? Protect Your Lab Investment and Data Integrity



















