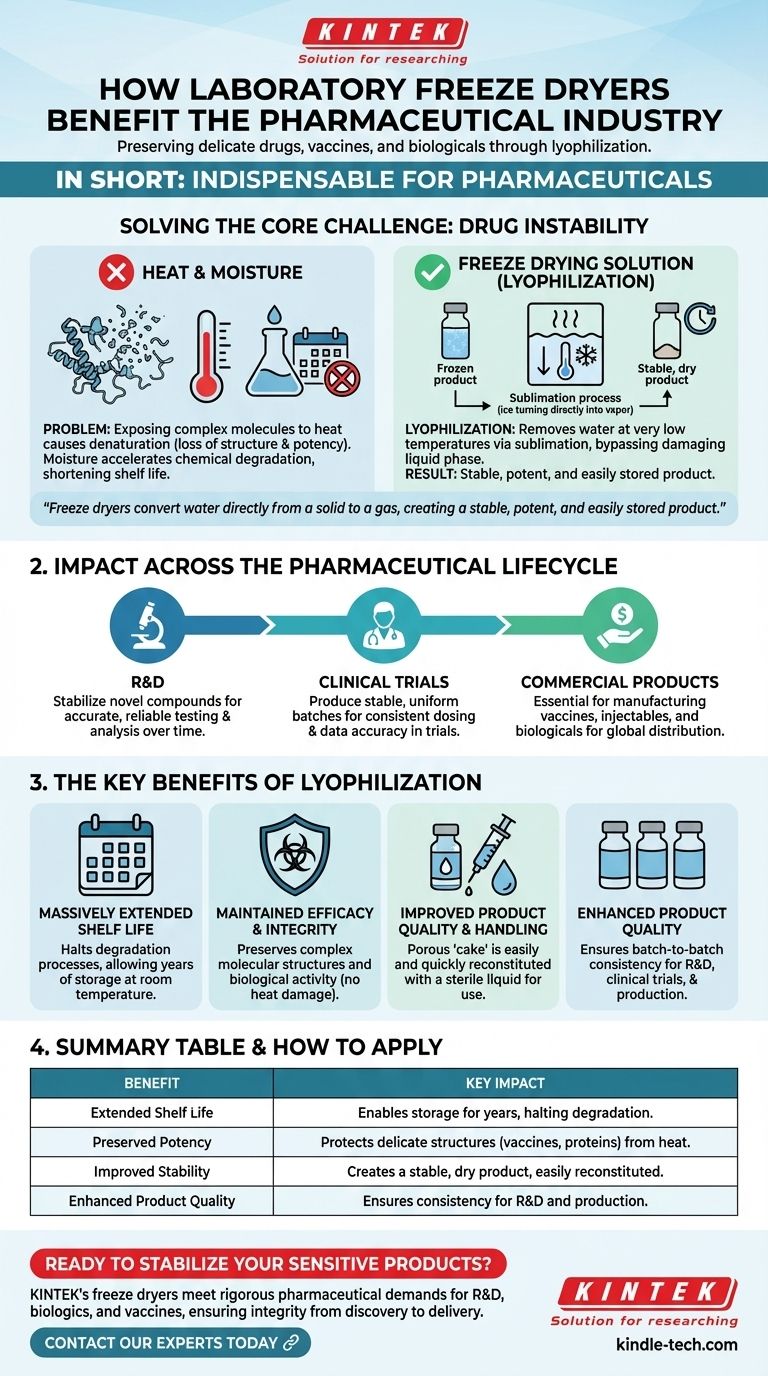
In short, laboratory freeze dryers are indispensable to the pharmaceutical industry for preserving delicate, heat-sensitive drugs, vaccines, and biologicals. This process, known as lyophilization, removes water at low temperatures, which dramatically extends the product's shelf life without compromising its chemical structure or therapeutic effectiveness.
The core challenge in modern drug development is the inherent instability of complex molecules like vaccines and proteins. Freeze dryers solve this by converting water directly from a solid to a gas, bypassing the damaging liquid phase and creating a stable, potent, and easily stored product.

Solving the Core Challenge: Drug Instability
The Problem with Heat and Moisture
Many of today's most advanced drugs—including vaccines, enzymes, antibiotics, and proteins—are highly sensitive.
Exposing these complex molecules to even moderate heat during traditional drying processes can cause them to denature, or lose their essential structure. This structural change results in a permanent loss of potency and efficacy.
Moisture also presents a significant threat, creating an environment where chemical degradation can occur, shortening the viable shelf life of a drug.
The Freeze Drying Solution
Freeze drying, or lyophilization, circumvents these issues by processing materials at very low temperatures.
The process involves first freezing the product, then placing it under a deep vacuum. This allows the frozen water to turn directly into vapor in a process called sublimation, completely bypassing the damaging liquid water phase.
The result is a dry, structurally sound product that is preserved in a state of suspended animation, protected from both thermal damage and moisture-driven degradation.
Impact Across the Pharmaceutical Lifecycle
Laboratory freeze dryers are not just a manufacturing tool; they are a critical technology used from initial discovery through to commercial production.
In Research and Development (R&D)
During the early stages of drug discovery, scientists use freeze dryers to stabilize new and often unstable drug candidates. This allows for accurate testing and analysis over time without the variable of product degradation.
During Clinical Trials
To ensure the integrity of a study, every dose of an investigational drug must be consistent. Freeze drying produces stable, uniform batches that can be reliably stored and administered to trial participants, guaranteeing data accuracy.
For Commercial Products
Freeze dryers are essential for manufacturing small batches of commercial products. This is especially true for vaccines, injectable formulations, and biological drugs that would otherwise be too unstable for storage and global distribution.
The Key Benefits of Lyophilization
The strategic application of freeze drying delivers several tangible advantages that are critical for pharmaceutical success.
Massively Extended Shelf Life
The primary benefit is a significant extension of product shelf life. By removing water, lyophilization halts most biological and chemical processes that cause degradation, allowing products to be stored for years at room temperature.
Maintained Efficacy and Integrity
Because the process avoids heat, the drug’s complex molecular structure and biological activity are preserved. This ensures that when the product reaches the patient, it delivers its intended therapeutic effect with full potency.
Improved Product Quality and Handling
The resulting freeze-dried "cake" is porous and easily reconstituted with a sterile liquid before administration. This improves the physical properties of the drug, making it simple to prepare for use in a clinical setting.
How to Apply This to Your Goal
The value of freeze drying depends on your specific objective within the pharmaceutical pipeline.
- If your primary focus is early-stage R&D: Freeze drying is your key to stabilizing novel compounds, ensuring you can conduct reliable and repeatable experiments on new drug candidates.
- If your primary focus is vaccine or biologic development: This technology is non-negotiable for preserving the intricate structure and potency of your products, making them viable for storage and distribution.
- If your primary focus is commercial drug stability: Lyophilization is the gold standard for creating formulations with a long shelf life, reducing waste and expanding the reach of sensitive medications.
Ultimately, freeze drying transforms fragile, short-lived medical innovations into stable, reliable, and life-saving products.
Summary Table:
| Benefit | Key Impact |
|---|---|
| Extended Shelf Life | Enables storage for years by removing water, halting degradation. |
| Preserved Potency | Protects delicate molecular structures (vaccines, proteins) from heat damage. |
| Improved Stability | Creates a stable, dry product that is easily reconstituted before use. |
| Enhanced Product Quality | Ensures batch-to-batch consistency for R&D, clinical trials, and commercial production. |
Ready to stabilize your sensitive pharmaceutical products?
KINTEK's laboratory freeze dryers are engineered to meet the rigorous demands of pharmaceutical R&D and production. Whether you are developing novel biologics, vaccines, or injectable drugs, our equipment ensures your products maintain their integrity and potency from discovery to delivery.
Contact our experts today to find the perfect lyophilization solution for your lab's needs and discover how we can help you achieve superior product stability and extended shelf life.
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Manual Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- How does an autoclave ensure the reliability of experimental results? Achieving a Sterile Baseline for Lab Research
- What experimental conditions do stainless steel autoclaves provide for PCT-A leaching? Optimize Phosphate Glass Testing
- Why is the prevention of air entrapment critical for the autoclave sterilization process? Ensure 100% Sterility Today
- What are the standard operating parameters for an autoclave? Master Temperature, Pressure, and Time for Sterilization
- What role does an autoclave play in remediation experiments? Ensure Precision by Eliminating Biological Noise



















