Annealing is a heat treatment process that fundamentally alters a material's microstructure to change its mechanical and physical properties. By heating a material to a specific temperature and then cooling it slowly, annealing generally makes a material softer, more ductile, and easier to work with. It also refines the crystal structure, which can improve properties like electrical conductivity.
The core purpose of annealing is to relieve internal stresses and reduce the density of crystal defects, known as dislocations. This controlled structural "reset" makes the material more uniform and predictable, though the exact outcome—such as an increase in strength versus an increase in softness—depends on the specific alloy and annealing parameters used.

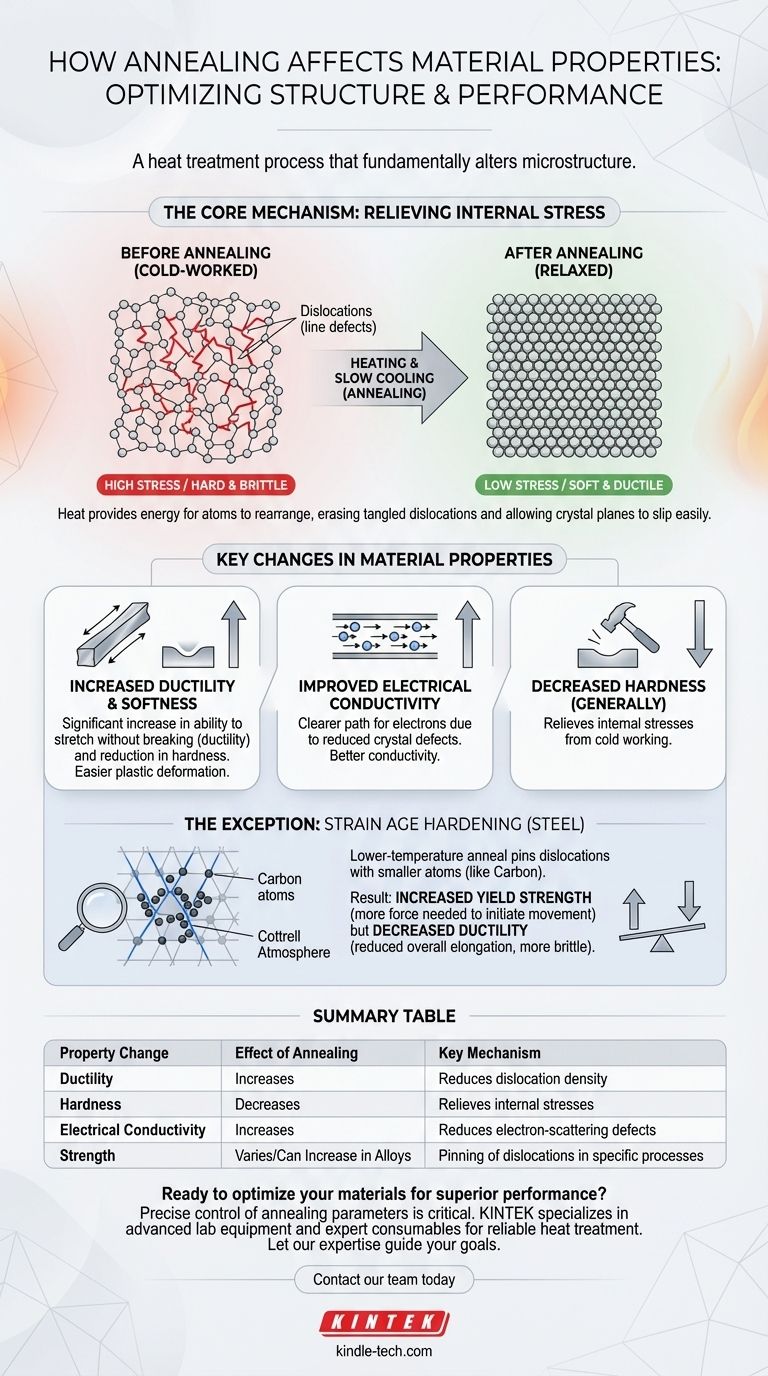
The Core Mechanism: Relieving Internal Stress
To understand annealing, you must first understand the microscopic imperfections that define a material's behavior.
What Are Dislocations?
Dislocations are line defects or irregularities within a material's crystal structure. They are naturally introduced during solidification or multiplied during manufacturing processes like rolling, forging, or bending (known as cold working).
These tangled dislocations are what make a cold-worked material hard and brittle. They impede the smooth sliding of crystal planes, which is necessary for the material to deform plastically.
How Heat Enables Change
Applying heat—the core of the annealing process—gives the atoms within the crystal lattice enough energy to vibrate and move.
This atomic mobility allows the microstructure to repair itself. Atoms can rearrange into a more orderly, lower-energy state, effectively "erasing" many of the dislocations created during cold working.
Key Changes in Material Properties
This internal rearrangement leads to several critical and desirable changes in the material's bulk properties.
Increased Ductility and Softness
The primary and most common result of annealing is a significant increase in ductility (the ability to be stretched without breaking) and a reduction in hardness.
With fewer dislocations to obstruct movement, the crystal planes can slip past one another more easily. This makes the material softer and capable of undergoing significant plastic deformation before fracturing.
Improved Electrical Conductivity
Dislocations in the crystal lattice act as scattering sites that impede the flow of electrons.
By reducing the density of these defects, annealing creates a clearer path for electrons to travel. This results in better electrical conductivity, a critical property for materials used in wiring and electronic components.
Understanding the Trade-offs and Specific Cases
While annealing is typically associated with softening, its effects can be more complex, particularly in specific alloys like steel.
The Exception: Strain Age Hardening
In certain cases, a lower-temperature anneal does not cause a full structural reset. Instead, it provides just enough energy for smaller atoms within the alloy, such as carbon in steel, to migrate.
The Cottrell Atmosphere Effect
These mobile carbon atoms are drawn to the strain fields surrounding existing dislocations, forming what is known as a Cottrell atmosphere.
This cloud of atoms effectively pins the dislocations in place, making it more difficult to initiate their movement.
The Result: Increased Strength, Decreased Ductility
Because more force is required to break the dislocations free from this pinning effect, the material's yield strength increases.
However, this comes at a cost. The process reduces the density of movable dislocations, which ultimately leads to a decrease in overall elongation and ductility, making the material more brittle. This is a crucial trade-off seen in specific steel treatments.
Making the Right Choice for Your Goal
Annealing is not a one-size-fits-all process. The desired outcome dictates the type of annealing required.
- If your primary focus is improving machinability or formability: A full anneal is used to achieve maximum softness and ductility by drastically reducing dislocation density.
- If your primary focus is optimizing electrical conductivity: Annealing is a critical step to minimize the crystal defects that impede electron flow.
- If your primary focus is a precise strength-to-toughness ratio in an alloy: A specific, lower-temperature process anneal may be used to intentionally pin dislocations, increasing yield strength at the expense of ductility.
Ultimately, understanding annealing empowers you to tailor a material’s internal structure to its exact intended function.
Summary Table:
| Property Change | Effect of Annealing | Key Mechanism |
|---|---|---|
| Ductility | Increases | Reduces dislocation density, allowing easier crystal plane slippage. |
| Hardness | Decreases (Generally) | Relieves internal stresses from cold working. |
| Electrical Conductivity | Increases | Reduces electron-scattering crystal defects. |
| Strength | Varies (Can Increase in Alloys) | In processes like strain age hardening, dislocations are pinned, increasing yield strength. |
Ready to optimize your materials for superior performance?
The precise control of annealing parameters is critical to achieving the exact balance of strength, ductility, and conductivity your application demands. KINTEK specializes in providing the advanced lab equipment and expert consumables needed for reliable and repeatable heat treatment processes.
Let our expertise guide your material science goals. Contact our team today to discuss how we can support your laboratory's specific needs.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- What is a vertical tube furnace? Leverage Gravity for Superior Uniformity and Process Control
- What is the temperature of a quartz tube furnace? Master the Limits for Safe, High-Temp Operation
- What is the difference between upflow and horizontal furnace? Find the Perfect Fit for Your Home's Layout
- How do you clean a tubular furnace tube? A Step-by-Step Guide to Safe and Effective Maintenance
- How do you clean a quartz tube furnace? Prevent Contamination & Extend Tube Lifespan



















