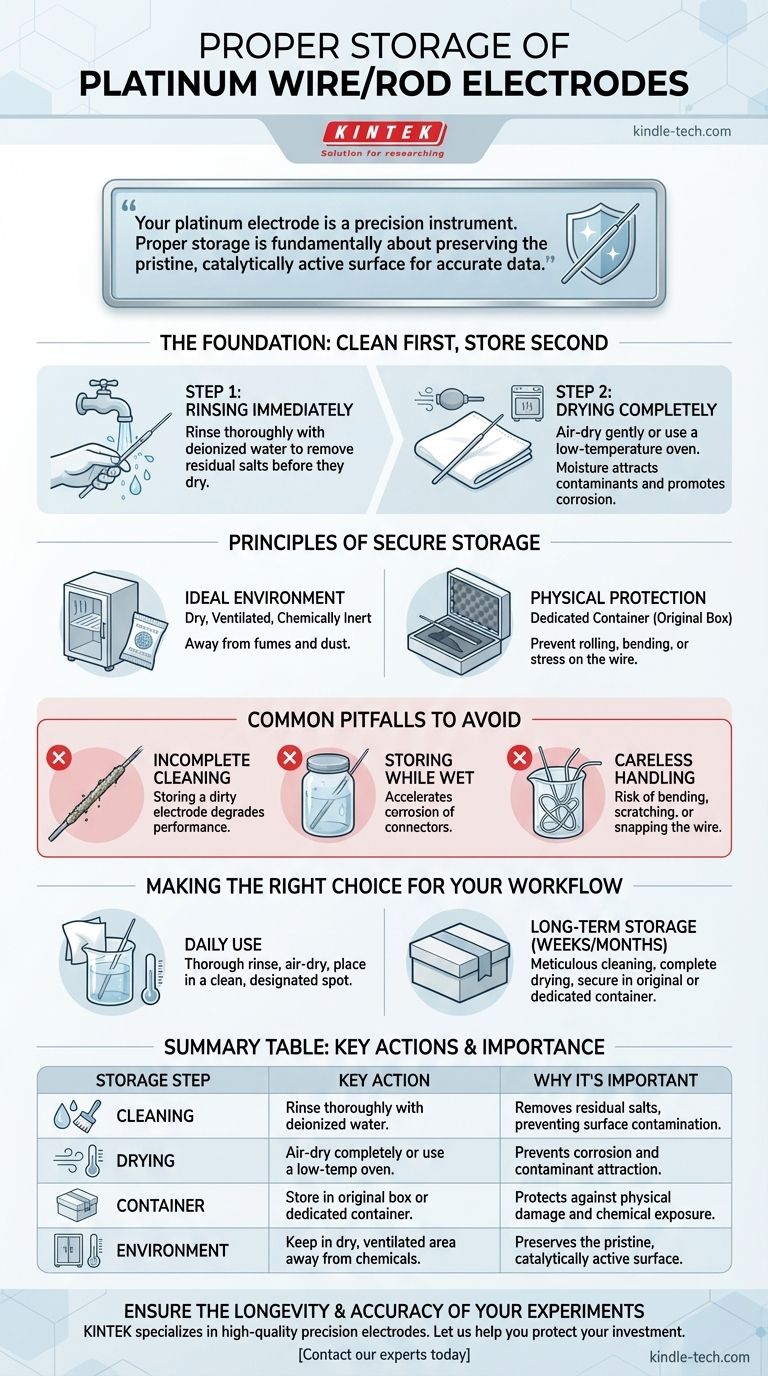
For proper storage, a platinum wire or rod electrode must first be thoroughly cleaned with deionized water and completely dried. It should then be placed in a dedicated, non-contaminating container—ideally its original box—and stored in a dry, ventilated area away from any corrosive chemicals to prevent both physical damage and surface contamination.
Your platinum electrode is a precision instrument, not just a piece of metal. Proper storage is fundamentally about preserving the pristine, catalytically active surface of the platinum, which is the single most important factor for obtaining accurate and reproducible experimental data.

The Foundation: Clean First, Store Second
Before you even consider storage, the electrode must be impeccably clean. Any residue left from an experiment can harden, corrode connections, or contaminate future work.
Rinsing Immediately After Use
As soon as an experiment is complete, remove the electrode from the electrolyte.
Rinse it thoroughly and repeatedly with deionized or distilled water. This simple step removes residual salts and substances before they have a chance to dry on the surface.
Drying Completely
Moisture attracts airborne contaminants and can promote corrosion on non-platinum components of the electrode assembly.
The electrode should be gently air-dried in a clean location or placed in a low-temperature oven. Ensure it is completely dry before moving it to its storage container.
Principles of Secure Electrode Storage
Once clean and dry, the goal of storage is to protect the electrode from two primary threats: chemical contamination and physical damage.
The Ideal Storage Environment
The storage location must be dry, ventilated, and chemically inert.
Avoid storing the electrode in the open on a lab bench where it can be exposed to dust or corrosive fumes from other chemicals. A dedicated cabinet or drawer is a much better choice.
Protecting Against Physical Damage
Platinum wires are delicate and the connection point between the wire and the electrode body is a common point of failure.
Always store the electrode in a dedicated container that prevents it from rolling or being struck by other objects. The original shipping box is designed for this purpose. If using another container, ensure the platinum element does not bear any weight or stress.
The Rationale: Why Surface Integrity Is Paramount
A platinum electrode's value lies in its inertness and its predictable catalytic surface.
Contamination, even on a microscopic level, can poison this surface, altering its electrochemical behavior. This directly leads to inaccurate measurements, poor reproducibility, and failed experiments. Physical scratches create surface inconsistencies with the same negative effect.
Common Pitfalls to Avoid
Mistakes in storage are common and can drastically shorten an electrode's effective lifespan. Understanding these pitfalls is key to preventing them.
Pitfall 1: Incomplete Cleaning
Simply dipping the electrode in water is not enough. A thorough rinse is required to remove all traces of the previous electrolyte. Storing a dirty electrode is the fastest way to degrade its performance.
Pitfall 2: Storing While Wet
Storing a damp electrode in a sealed container creates a humid micro-environment. This can accelerate corrosion of the pin connector or other non-platinum parts, leading to poor electrical conductivity.
Pitfall 3: Careless Physical Handling
Never let the electrode roll around in a drawer or toss it into a beaker with other equipment. The platinum wire can easily be bent, scratched, or snapped off from its mounting. Always handle it with deliberate care.
Making the Right Choice for Your Workflow
Your storage protocol can be adapted slightly based on how frequently you use the electrode.
- If you are using the electrode daily: A thorough rinse and air-dry, followed by placement in a clean, designated spot on your workstation (like a beaker with a soft tissue at the bottom), is sufficient.
- If you are storing the electrode long-term (weeks or months): You must follow the full protocol of meticulous cleaning, complete drying, and secure placement in its original box or a dedicated, sealed container.
Ultimately, treating your electrode with care is a direct investment in the quality and reliability of your scientific work.
Summary Table:
| Storage Step | Key Action | Why It's Important |
|---|---|---|
| Cleaning | Rinse thoroughly with deionized water immediately after use. | Removes residual salts and substances that can contaminate the surface. |
| Drying | Air-dry completely or use a low-temperature oven. | Prevents corrosion of non-platinum parts and avoids attracting contaminants. |
| Container | Store in the original box or a dedicated, non-contaminating container. | Protects against physical damage (bending, scratching) and chemical exposure. |
| Environment | Keep in a dry, ventilated area away from corrosive chemicals. | Preserves the pristine, catalytically active platinum surface for accurate data. |
Ensure the Longevity and Accuracy of Your Experiments
Your platinum electrodes are precision instruments critical for reproducible results. Proper storage is non-negotiable for maintaining their performance.
KINTEK specializes in high-quality lab equipment and consumables, including precision electrodes. We understand that your research depends on reliable tools. Let us help you protect your investment.
Contact our experts today to discuss your laboratory needs and discover how our products and support can enhance the reliability and efficiency of your work.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
People Also Ask
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs



















