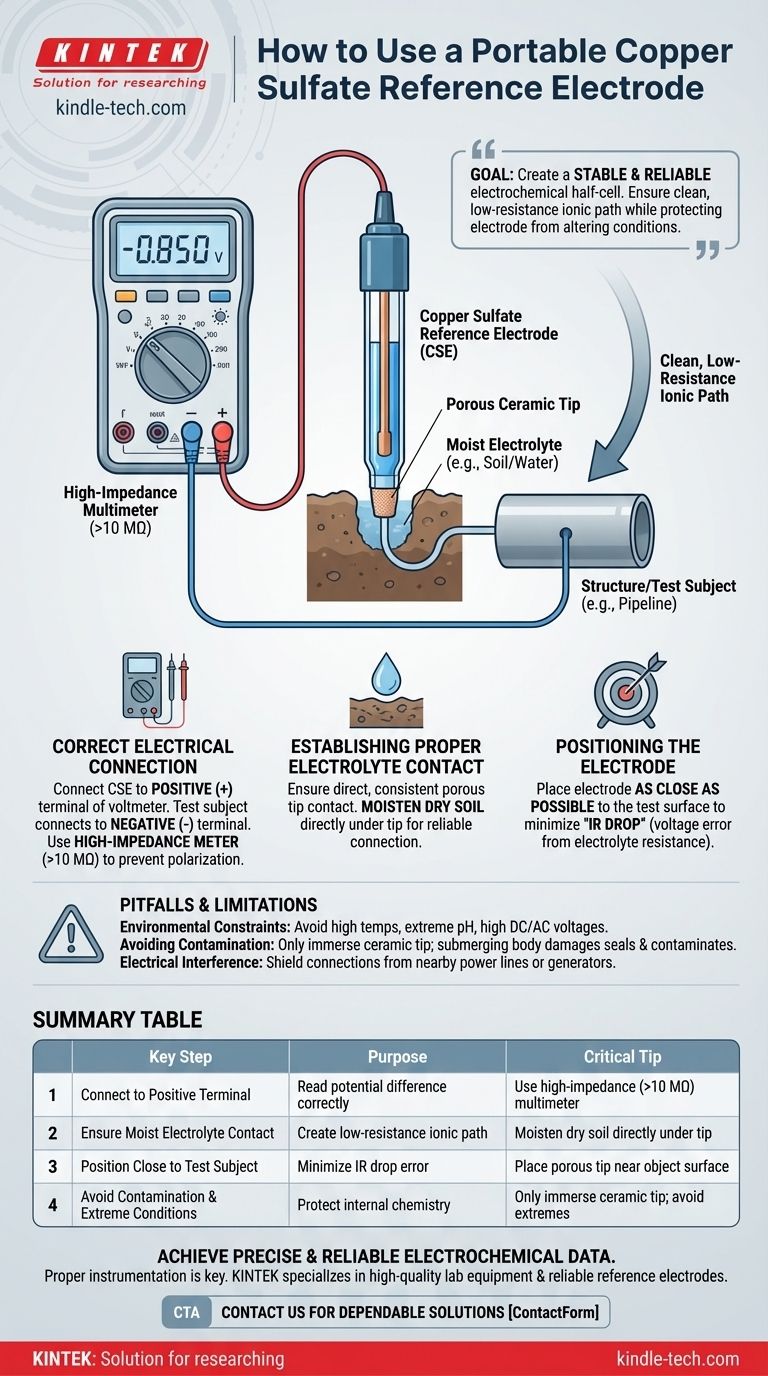
To use a portable copper sulfate reference electrode correctly, you must connect its lead wire to the positive (+) terminal of a high-impedance multimeter or potentiometer. The structure or other electrode you are measuring is then connected to the negative (-) terminal. Insert the porous tip of the reference electrode into the electrolyte (e.g., water or soil), ensuring there is a solid, moist connection to establish a proper electrical circuit.
The goal is not just to connect the electrode, but to create a stable and reliable electrochemical half-cell. Success depends on establishing a clean, low-resistance ionic path to the electrolyte while protecting the electrode from conditions that would alter its stable reference potential.

The Fundamentals of a Stable Measurement
A reference electrode provides a stable, known potential, acting as a reliable zero-point for your measurements. Every step in its use is designed to preserve that stability.
Correct Electrical Connection
Connect the copper sulfate electrode (CSE) to the positive terminal of your voltmeter. The test subject, such as a steel pipeline or a lab sample, connects to the negative terminal.
This setup allows for a direct reading of the potential difference. For example, in cathodic protection, structures are maintained at a negative potential, and this connection will show that value directly.
Crucially, always use a high-impedance multimeter (typically >10 MΩ). A low-impedance meter would draw current through the reference electrode, polarizing it and permanently altering its potential, rendering the measurement invalid.
Establishing Proper Electrolyte Contact
The electrode's porous tip must make direct and consistent contact with the electrolyte. This creates the necessary path for ion flow, completing the circuit.
For measurements in soil, the soil must be moist. Dry soil is a poor conductor and will result in an unstable or nonexistent reading. If the soil is dry, carefully moisten the area directly under the tip with a small amount of water.
Positioning the Electrode
To get the most accurate reading, place the reference electrode as close as possible to the surface of the object you are measuring.
This minimizes the "IR drop," which is a voltage error caused by the electrical resistance of the electrolyte itself. Placing the electrode far away can introduce this error, skewing your results.
Understanding the Pitfalls and Limitations
Improper use can not only ruin your data but also damage the electrode. Being aware of its operational limits is essential for reliable work.
Environmental Constraints
A CSE's stability is dependent on its internal chemistry. Extreme conditions can disrupt this equilibrium and invalidate your readings.
Avoid using the electrode in environments with:
- Excessively high temperatures
- Highly acidic or highly alkaline pH levels
- High DC or AC voltages
These factors can permanently change the electrode's reference potential. It is best to conduct measurements under normal temperature and pressure.
Avoiding Contamination
Only the porous ceramic tip of the electrode should be immersed in the electrolyte.
Submerging the electrode body can damage the seals over time. This can lead to the electrolyte contaminating the internal copper sulfate solution or the internal solution leaking out, both of which will destroy the electrode's accuracy.
Electrical Interference
External electrical fields can introduce noise and error into your sensitive potential measurements.
Be aware of interference from nearby sources like power lines, electric generators, or other strong electromagnetic fields. If readings are unstable, try to shield your connections or take measurements when interfering equipment is turned off.
Checklist for Reliable Measurements
Your approach should adapt slightly based on your primary objective.
- If your primary focus is accurate cathodic protection surveys: Place the electrode tip on the soil surface directly above the pipeline or structure and ensure consistent soil moisture to get a true, IR-drop-minimized potential reading.
- If your primary focus is repeatable lab experiments: Position the electrode at a consistent distance from your sample in every test and ensure the electrolyte is free from contaminants that could affect the electrode.
- If your primary focus is equipment longevity: Always check that the internal solution is saturated (visible copper sulfate crystals) before use and store the electrode upright with its protective cap on to prevent drying or leakage.
A clear and stable connection is the foundation of every trustworthy electrochemical measurement.
Summary Table:
| Key Step | Purpose | Critical Tip |
|---|---|---|
| Connect to Positive Terminal | To read the potential difference correctly. | Use a high-impedance (>10 MΩ) multimeter to prevent polarization. |
| Ensure Moist Electrolyte Contact | To create a low-resistance ionic path for a stable circuit. | Moisten dry soil directly under the tip for a reliable connection. |
| Position Close to Test Subject | To minimize measurement error caused by electrolyte resistance (IR drop). | Place the porous tip as near as possible to the object's surface. |
| Avoid Contamination & Extreme Conditions | To protect the electrode's internal chemistry and reference potential. | Only immerse the ceramic tip; avoid high temperatures, extreme pH, and strong voltages. |
Achieve precise and reliable electrochemical data in your lab or field work. Proper instrumentation is key to success. KINTEK specializes in high-quality lab equipment and consumables, including reliable reference electrodes and measurement tools. Our experts can help you select the right equipment for your specific application, ensuring accurate results every time.
Contact us today to discuss your laboratory needs and how we can support your research with dependable solutions. #ContactForm
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
People Also Ask
- How should a copper sulfate reference electrode be maintained? Ensure Accurate Electrochemical Measurements
- What are the advantages and disadvantages of the ceramic core type copper sulfate reference electrode?
- What is the operating principle of a copper sulfate reference electrode? Reliable Potential Measurement Explained
- What are the pre-treatment steps before using a portable copper sulfate reference electrode? Ensure Accurate Corrosion Potential Measurements
- What precautions should be taken when handling and using a copper sulfate reference electrode? Ensure Accurate Electrochemical Measurements



















