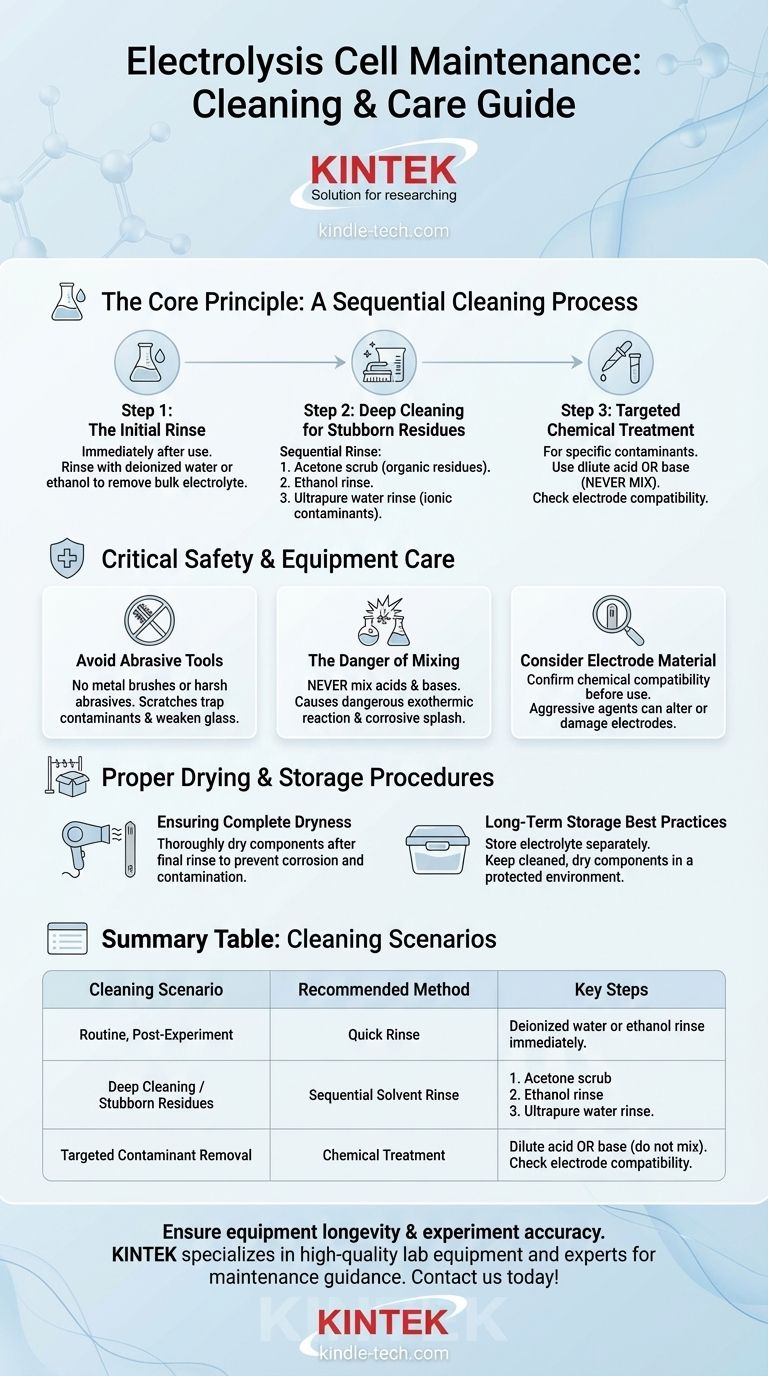
For routine maintenance, an electrolysis cell and its electrodes should be cleaned immediately after use with deionized water or ethanol. For more stubborn residues or previously used equipment, a sequential rinse using acetone, then ethanol, and finally high-purity water is the most effective method. In specific cases, a dilute acid or base can be used, but never together.
The core objective of cleaning an electrolysis cell is not just visible cleanliness, but the complete removal of chemical residues to prevent cross-contamination between experiments. This is achieved through a systematic solvent rinsing process and careful handling to protect the integrity of the cell and electrodes.

The Core Principle: A Sequential Cleaning Process
Proper cleaning is a multi-step process designed to systematically dissolve and remove different types of potential contaminants. Acting immediately after an experiment is critical, as it prevents residues from drying and adhering strongly to the surfaces.
Step 1: The Initial Rinse
For routine cleaning right after an experiment, a simple rinse is often sufficient. Use deionized water or ethanol to wash the reaction vessel and the electrodes. This removes the bulk of the electrolyte and any loosely adhered products.
Step 2: Deep Cleaning for Stubborn Residues
If the cell has been sitting or contains more persistent contamination, a more rigorous solvent sequence is required. First, scrub the inner walls with acetone to dissolve organic residues. Follow this with an ethanol rinse, and finish with a final, thorough rinse using ultrapure water (resistivity > 18.2 MΩ・cm) to remove any remaining ionic contaminants.
Step 3: Targeted Chemical Treatment
For specific, known contaminants that solvents cannot remove, a targeted chemical treatment may be necessary. A dilute acid or base can be used for this purpose. The choice depends entirely on the nature of the residue you are trying to remove.
Critical Safety and Equipment Care
Improper cleaning techniques can be more damaging than no cleaning at all. Following strict safety protocols is non-negotiable to protect both the user and the expensive equipment.
Avoid Abrasive Tools
Never use metal brushes or other hard, abrasive tools to scrub the cell. These instruments will scratch the glass surfaces of the vessel, creating sites where contaminants can become trapped and potentially weakening the structural integrity of the cell.
The Danger of Mixing Cleaning Agents
Under no circumstances should you mix acids and bases (e.g., nitric acid and sodium hydroxide) in an attempt to create a stronger cleaning solution. This will cause a dangerous exothermic reaction, which can generate intense heat, splash corrosive chemicals, and damage the equipment.
Consider Electrode Material
Before using a dilute acid or base, you must confirm that it is compatible with your electrode material. Aggressive chemical agents can corrode or permanently damage the electrode surface, altering its electrochemical properties and ruining future experiments.
Proper Drying and Storage Procedures
The maintenance process isn't complete until the components are safely stored. Proper drying and storage are essential for long-term reliability.
Ensuring Complete Dryness
After the final rinse, the electrodes and reaction vessel must be dried thoroughly before storage. Moisture can lead to corrosion or provide an environment for contaminants to settle on the surfaces.
Long-Term Storage Best Practices
For long-term storage, always pour the electrolyte out of the cell and store it in a separate, sealed container. The cleaned and dried cell components should then be stored in a dry, protected environment to prevent dust accumulation and moisture exposure.
Matching Your Cleaning Protocol to Your Needs
Your cleaning strategy should adapt to the situation. A blanket approach is inefficient and can be insufficient for ensuring the quality of your results.
- If your primary focus is routine, post-experiment cleaning: An immediate and thorough rinse with deionized water or ethanol is sufficient to prepare for the next run.
- If your primary focus is restoring a heavily used or contaminated cell: Execute the full sequential cleaning process: acetone scrub, followed by ethanol and ultrapure water rinses.
- If your primary focus is long-term storage or system shutdown: Perform a full deep cleaning, ensure all components are completely dry, and store the electrolyte separately in a sealed container.
A disciplined and appropriate cleaning regimen is the foundation of reliable and repeatable electrochemical results.
Summary Table:
| Cleaning Scenario | Recommended Method | Key Steps |
|---|---|---|
| Routine, Post-Experiment | Quick Rinse | Deionized water or ethanol rinse immediately after use. |
| Deep Cleaning / Stubborn Residues | Sequential Solvent Rinse | 1. Acetone scrub 2. Ethanol rinse 3. Ultrapure water rinse. |
| Targeted Contaminant Removal | Chemical Treatment | Use a dilute acid OR base (never mix), ensuring electrode compatibility. |
Ensure the longevity of your lab equipment and the accuracy of your electrochemical experiments. KINTEK specializes in high-quality lab equipment and consumables, serving all your laboratory needs. Our experts can help you select the right equipment and provide guidance on best practices for maintenance. Contact us today to learn how we can support your lab's success!
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Electrolytic Electrochemical Cell for Coating Evaluation
- Flat Corrosion Electrolytic Electrochemical Cell
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
People Also Ask
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- What are the necessary steps to prepare an all-quartz electrolytic cell before an experiment? Ensure Accuracy and Safety



















