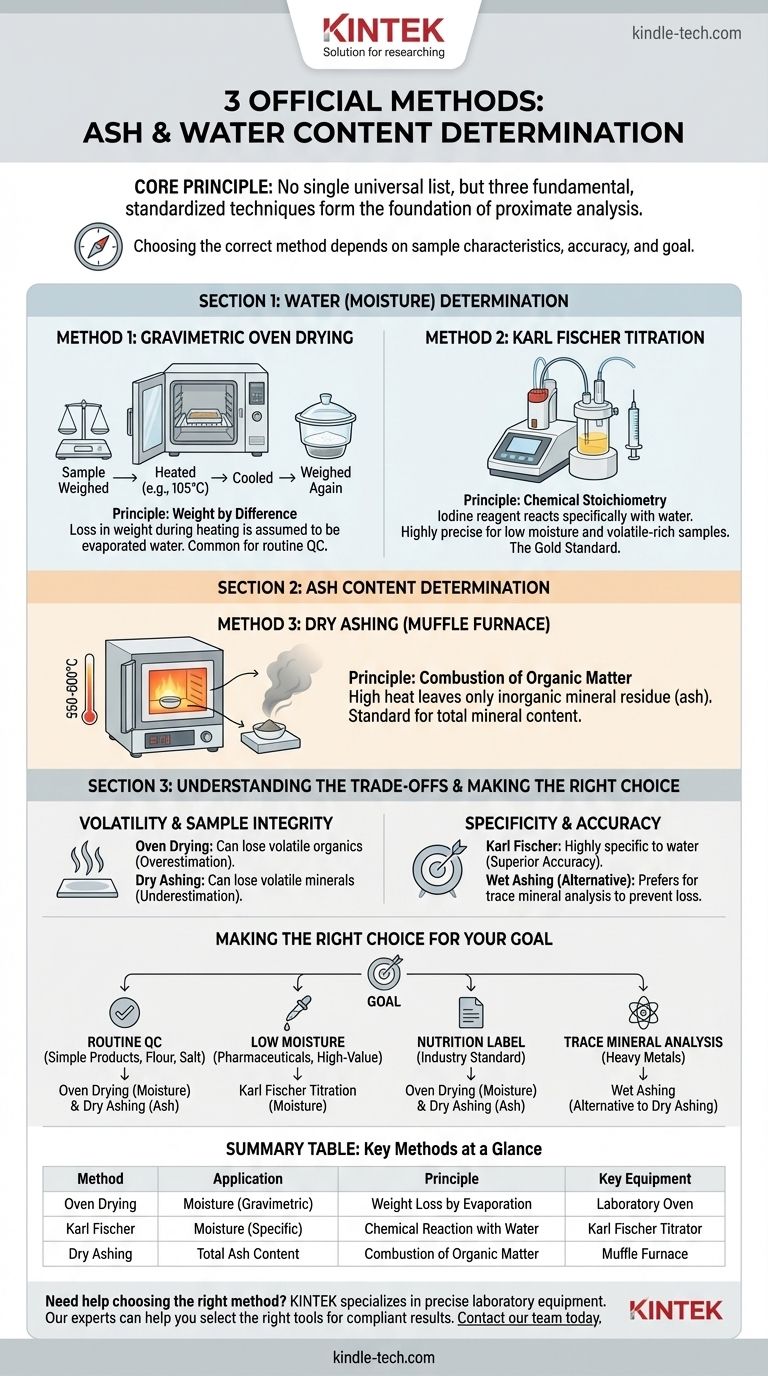
While no single list of "3 official methods" exists across all regulatory bodies, the most fundamental and universally recognized techniques for determining water and ash content are oven drying for moisture, dry ashing using a muffle furnace for total ash, and the Karl Fischer titration for a more precise measurement of water. These methods, standardized by organizations like AOAC International, form the foundation of proximate analysis in food science, agriculture, and quality control.
The core principle is not just to follow a method, but to choose the correct one. The choice between thermal (oven drying, ashing) and chemical (Karl Fischer) techniques depends entirely on your sample's characteristics, the required accuracy, and the specific question you are trying to answer.

Understanding Water (Moisture) Determination
Measuring water content is one of the most common procedures in analytical chemistry. It is critical for determining product quality, shelf stability, and nutritional labeling.
Method 1: Gravimetric Oven Drying
This is the most common and straightforward method for determining moisture content. A sample is weighed, heated in an oven at a specific temperature (e.g., 105°C) for a set time, cooled in a desiccator, and weighed again.
The principle is weight by difference. The loss in weight during heating is assumed to be entirely due to the evaporation of water. While simple and effective for many materials, it has limitations.
Method 2: Karl Fischer Titration
This is a highly accurate and specific chemical method that measures water directly. It is considered the gold standard for moisture analysis, especially for products with very low water content or those containing volatile compounds other than water.
The principle is chemical stoichiometry. A reagent containing iodine reacts specifically with water in the sample. The amount of reagent consumed is directly proportional to the amount of water present, providing a far more precise result than thermal methods.
Understanding Ash Content Determination
Ash refers to the inorganic mineral residue left after all organic matter has been completely burned away. This measurement is a proxy for the total mineral content of a sample.
Method 3: Dry Ashing (Muffle Furnace)
This is the standard method for determining total ash. A sample is weighed into a crucible and placed in a high-temperature muffle furnace (typically 550-600°C).
The extreme heat causes all organic components—proteins, fats, and carbohydrates—to combust and oxidize into gaseous products, leaving only the non-combustible inorganic minerals behind. The remaining residue is the ash.
Understanding the Trade-offs
Choosing a method is a technical decision with clear consequences for your results. You must understand the trade-offs between the primary techniques.
Volatility and Sample Integrity
Oven drying can inadvertently remove more than just water. Volatile organic compounds, such as flavor essences or short-chain fatty acids, can also evaporate, leading to an overestimation of moisture content.
Similarly, dry ashing can cause certain minerals like chlorides and nitrates to vaporize at high temperatures, leading to an underestimation of the true ash content.
Specificity and Accuracy
Karl Fischer titration is highly specific to water. It will not react with other volatile compounds, making it exceptionally accurate for samples where oven drying would be misleading (e.g., foods rich in essential oils or alcohols).
For ash, an alternative method called wet ashing uses strong acids and oxidizers to digest the organic matter at lower temperatures. This is slower and more hazardous but is preferred when you need to prevent the loss of volatile minerals before performing specific elemental analysis (e.g., testing for lead or selenium).
Speed and Equipment
Oven drying and dry ashing are simple and allow for high throughput with basic laboratory equipment. However, they are slow, often requiring several hours or even overnight runs.
Karl Fischer titration is much faster per sample (often just a few minutes) but requires a specialized and more expensive titrator instrument.
Making the Right Choice for Your Goal
Your analytical goal dictates the correct method. Use this guide to make a sound decision.
- If your primary focus is routine QC for a simple, stable product (like flour or salt): Oven drying for moisture and dry ashing for ash are perfectly suitable, cost-effective, and reliable.
- If your primary focus is measuring low moisture in a high-value product (like pharmaceuticals or specialty chemicals): Karl Fischer titration is the only method that provides the necessary accuracy and specificity.
- If your primary focus is creating a standard nutrition label: The conventional methods of oven drying and dry ashing are the industry standard and are legally defensible for reporting moisture and total mineral content.
- If your primary focus is preparing a sample for trace mineral analysis (e.g., heavy metals): Wet ashing is the superior choice to ensure volatile minerals are not lost before subsequent analysis by techniques like atomic absorption spectroscopy.
Ultimately, selecting the proper analytical method is the first and most critical step in generating data you can trust.
Summary Table:
| Method | Application | Principle | Key Equipment |
|---|---|---|---|
| Oven Drying | Moisture Content (Gravimetric) | Weight Loss by Evaporation | Laboratory Oven |
| Karl Fischer Titration | Moisture Content (Specific) | Chemical Reaction with Water | Karl Fischer Titrator |
| Dry Ashing | Total Ash Content | Combustion of Organic Matter | Muffle Furnace |
Need help choosing the right method for your lab's moisture or ash analysis?
KINTEK specializes in supplying the precise laboratory equipment you need—from durable muffle furnaces for dry ashing to reliable ovens and consumables. Our experts can help you select the right tools to ensure accurate, compliant results for your quality control, food science, or agricultural testing.
Contact our team today to discuss your application and get a personalized recommendation!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the use of muffle oven in laboratory? For Clean, High-Temperature Material Processing
- What is the difference between a muffle furnace and an incubator? Choose the Right Tool for Your Lab
- Which material is used in a muffle furnace? The Key to High-Temperature Performance and Purity
- How do the properties of materials change with the heat treatment? Tailor Hardness, Strength, and Ductility
- How do you prepare samples for IR? A Guide to Solid, Liquid, and Gas Sample Prep



















