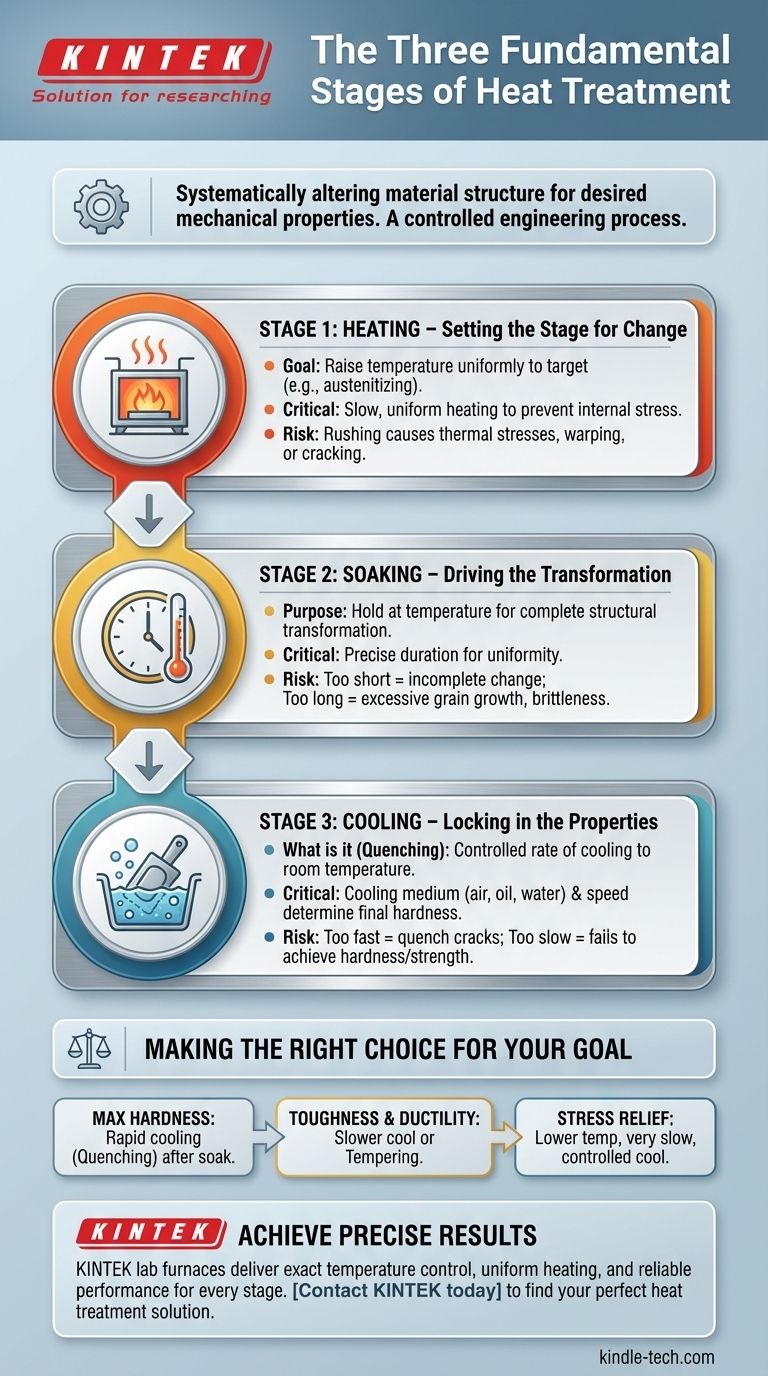
At its core, the heat treatment process consists of three fundamental stages. These are the Heating stage, where the material's temperature is uniformly raised; the Soaking stage, where it is held at that temperature for a specific duration; and the Cooling stage, where it is brought back to room temperature at a controlled rate. Each stage serves a distinct purpose in systematically altering the material's internal structure to achieve desired mechanical properties.
Heat treatment is not simply about changing a material's temperature. It is a highly controlled engineering process designed to manipulate a material's microscopic crystal structure to achieve specific, predictable outcomes like increased hardness, improved toughness, or relieved internal stress.

Stage 1: The Heating Cycle - Setting the Stage for Change
The initial heating phase is the foundation upon which the entire treatment is built. Its success depends entirely on precision and control.
The Goal of Heating
The primary objective is to raise the temperature of the entire component to a specific target, known as the austenitizing temperature in steels. This is the temperature at which the material's internal crystal structure becomes unstable and is ready to transform.
Why Uniformity is Critical
The heating must be slow and uniform enough to ensure the core of the material reaches the same temperature as the surface. Uneven heating can cause internal stresses, leading to warping or even cracking of the part.
Stage 2: The Soaking Period - Driving the Transformation
Once the material reaches the target temperature, it enters the soaking, or holding, phase. This is where the true metallurgical change occurs.
The Purpose of Soaking
The component is held at the specific temperature for a predetermined amount of time. This period allows the internal crystalline structure to fully and uniformly transform into a new structure (e.g., austenite in steel).
How Time Affects the Outcome
The duration of the soak is critical. A soak that is too short will result in an incomplete transformation and inconsistent properties. A soak that is too long can cause undesirable effects like excessive grain growth, which can make the material brittle.
Stage 3: The Cooling Phase - Locking in the Properties
The final cooling stage is arguably the most critical, as the rate of cooling directly determines the final mechanical properties of the material.
What is Cooling (Quenching)?
This stage involves rapidly or slowly reducing the material's temperature back to room temperature. The method and medium used—such as air, oil, water, or brine—are chosen specifically to control the cooling rate.
Why the Cooling Rate is Everything
The speed of cooling "locks in" a specific crystal structure. A very fast cool (a quench) traps a hard, brittle structure like martensite. A slower cool allows softer, more ductile structures like pearlite or bainite to form. This control is how metallurgists can produce a wide range of properties from the same base material.
Understanding the Trade-offs and Risks
Each stage of heat treatment presents opportunities for error. Understanding these risks highlights the importance of precise control over temperature, time, and atmosphere (such as a vacuum).
The Risk of Improper Heating
Rushing the heating cycle is a common mistake. This can create a significant temperature differential between the surface and the core of the part, generating thermal stresses that cause distortion.
The Danger of Incorrect Soaking
The soaking period is a delicate balance. Insufficient time leads to a non-uniform structure and unreliable performance. Excessive time can make the final product weak and brittle, even if it is hard.
The Consequence of Poor Cooling
The wrong cooling rate is the most common cause of failure. Cooling too fast can cause quench cracks and extreme brittleness. Cooling too slowly will fail to achieve the desired hardness and strength.
Making the Right Choice for Your Goal
The parameters for each of the three stages are selected based on the desired final properties of the component.
- If your primary focus is maximum hardness: The goal is a rapid cooling (quenching) phase after a proper heating and soaking cycle to form a fully martensitic structure.
- If your primary focus is toughness and ductility: The goal involves a slower cooling rate or a secondary heat treatment process (like tempering) to refine the brittle structure into a stronger, more resilient one.
- If your primary focus is stress relief: The goal requires a much lower heating temperature and a very slow, controlled cooling cycle to allow internal stresses to relax without changing the core hardness.
Ultimately, mastering the interplay between heating, soaking, and cooling is what transforms a simple piece of metal into a high-performance engineering component.
Summary Table:
| Stage | Key Objective | Critical Factor |
|---|---|---|
| 1. Heating | Uniformly raise the material to a target temperature. | Controlled, even heating to prevent warping/cracking. |
| 2. Soaking | Hold at temperature for complete microstructural transformation. | Precise duration to ensure uniformity and prevent grain growth. |
| 3. Cooling | Control the rate of cooling to lock in desired properties. | Cooling medium (air, oil, water) and speed determine final hardness/toughness. |
Achieve precise and repeatable results for your laboratory's heat treatment processes.
KINTEK specializes in high-quality lab furnaces and equipment that deliver the exact temperature control, uniform heating, and reliable performance required for every stage of heat treatment. Whether you are developing new alloys, processing samples for material science, or ensuring quality control, our solutions help you achieve the desired material properties—from maximum hardness to improved toughness—with confidence.
Contact KINTEK today to discuss your specific application and find the perfect heat treatment solution for your laboratory.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the most common source of heat used for brazing? Torch Brazing Explained for Optimal Joints
- How much does biochar pyrolysis cost? Unpacking the $230/Ton Production Price
- What is the difference between a vacuum pump and a regular pump? A Guide to Push vs. Pull Mechanics
- What is the purpose of the slow cooling (annealing) process for Ni-TiO2? Ensure Material Stability and Performance
- What is the function of a laboratory shaker in evaluating Fe-C@C nanoparticles? Optimize Methylene Blue Adsorption
- What is a membrane filter press for wastewater treatment? Achieve Drier Sludge & Lower Disposal Costs
- Why Use HIP or SPS After Mechanical Alloying of Alloys? Achieve Full Density and Structural Integrity
- What are the fundamental steps of the sintering process? A Guide to Precision Powder Metallurgy



















