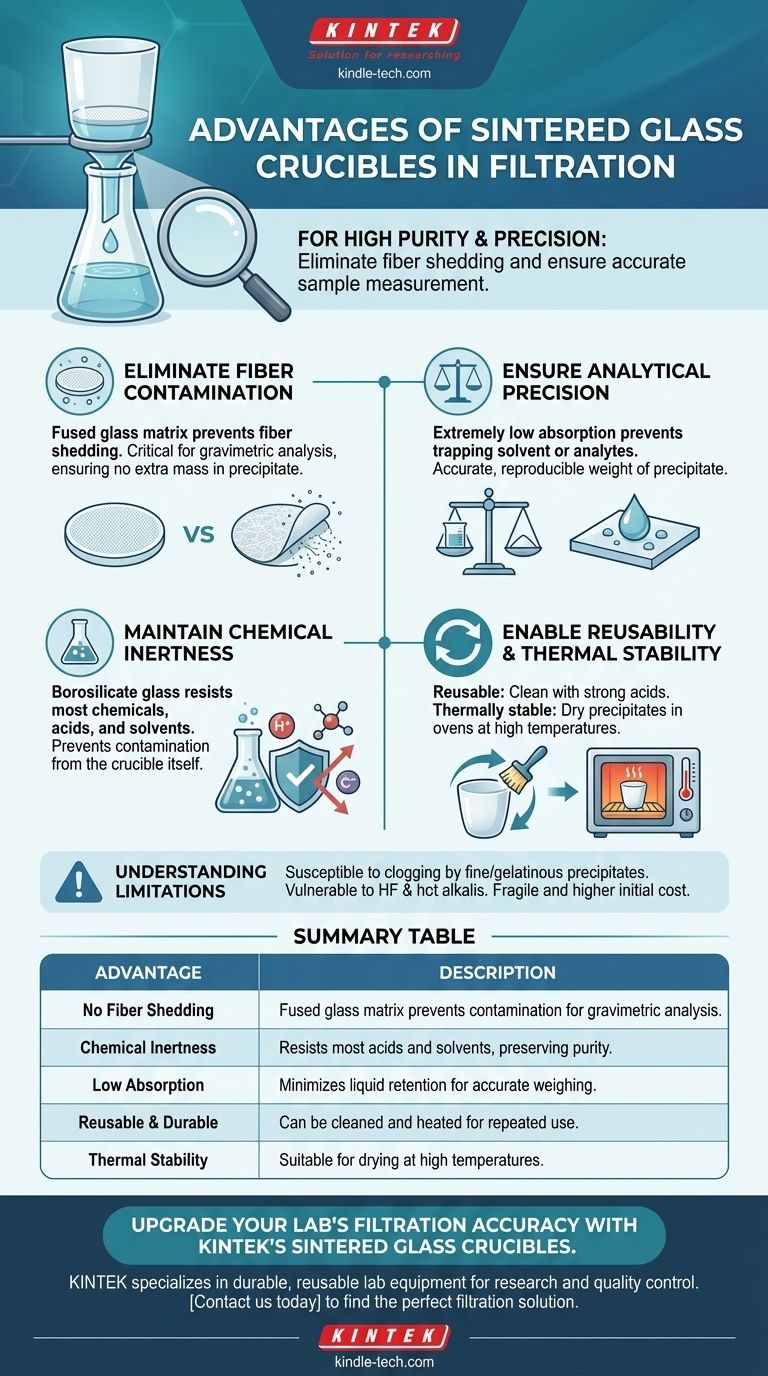
For any filtration task demanding high purity and precision, a sintered glass crucible offers distinct advantages over traditional methods like filter paper. Its primary benefits are the complete elimination of fiber shedding from the filtration medium, exceptionally low absorption of the solution, and its chemically inert nature, which collectively ensure the integrity and accurate measurement of your sample.
The core advantage of a sintered glass crucible is not just its ability to filter, but its ability to do so with exceptional purity and reusability. It provides a clean, non-reactive, and stable system that is essential for precise quantitative chemical analysis.

Why Sintered Glass Excels in Filtration
A sintered glass crucible, also known as a fritted glass crucible, has a porous glass disc fused into its base. This integrated design is the source of its primary benefits.
Eliminating Fiber Contamination
Unlike paper or fiber-based filters, the sintered glass disc is a solid, fused matrix of glass particles. This means it cannot shed fibers into the filtrate.
This is critical in applications like gravimetric analysis, where even microscopic fibers from filter paper would add to the measured mass of the precipitate, leading to inaccurate results.
Ensuring Analytical Precision
The borosilicate glass construction has extremely low absorption properties. The material does not trap or retain significant amounts of the solvent or dissolved analytes.
When you are trying to collect and weigh a precipitate, this ensures that the final weight is not artificially inflated by absorbed liquid, leading to more accurate and reproducible measurements.
Maintaining Chemical Inertness
Sintered glass crucibles are typically made from borosilicate glass, which is highly resistant to most chemicals, including strong acids and solvents.
This inertness prevents the crucible itself from reacting with or leaching impurities into your solution, preserving the purity of both the solid precipitate and the liquid filtrate.
Enabling Reusability and Thermal Stability
A key practical advantage is that these crucibles are reusable. They can be cleaned effectively with strong acids (like chromic or nitric acid) to remove residual precipitates.
Furthermore, they are thermally stable and can be heated in an oven to high temperatures to dry a collected precipitate to a constant weight, a standard procedure in gravimetric analysis.
Understanding the Trade-offs and Limitations
While powerful, sintered glass crucibles are not the perfect solution for every scenario. Understanding their limitations is key to using them effectively.
Susceptibility to Clogging
The fine pores of the sintered disc can be easily clogged by very fine or gelatinous precipitates, such as aluminum hydroxide. Once clogged, they can be extremely difficult or impossible to clean completely, rendering the crucible unusable.
Chemical Vulnerability
Despite their general inertness, sintered glass is attacked by hydrofluoric acid and hot, strong alkaline solutions. Using these chemicals will damage the porous disc and destroy the crucible.
Fragility and Upfront Cost
Being made of glass, these crucibles are inherently fragile and can break if dropped or subjected to thermal shock. They also represent a higher initial investment compared to a simple funnel and disposable filter papers.
Making the Right Choice for Your Goal
Selecting the correct filtration tool depends entirely on the requirements of your specific procedure.
- If your primary focus is quantitative gravimetric analysis: The sintered glass crucible is the superior choice due to its precision, purity, and lack of fiber shedding.
- If your primary focus is filtering very fine or gelatinous precipitates: A Büchner funnel with appropriate filter paper may be a more practical option to avoid irreversible clogging.
- If your primary focus is general, non-critical separation on a budget: Standard filter paper remains the most cost-effective and straightforward method.
Choosing the right tool is the first step toward ensuring the integrity and accuracy of your analytical work.
Summary Table:
| Advantage | Description |
|---|---|
| No Fiber Shedding | Fused glass matrix prevents contamination of the filtrate, crucial for gravimetric analysis. |
| Chemical Inertness | Resists most acids and solvents, preserving sample purity. |
| Low Absorption | Minimizes liquid retention for accurate precipitate weighing. |
| Reusable & Durable | Can be cleaned and heated for repeated use, offering long-term value. |
| Thermal Stability | Suitable for drying precipitates at high temperatures. |
Upgrade your lab's filtration accuracy with KINTEK's sintered glass crucibles.
Our high-quality borosilicate glass crucibles are designed to deliver the purity and precision your quantitative analysis demands. Eliminate fiber contamination and ensure chemically inert filtration for reliable, reproducible results.
KINTEK specializes in supplying durable, reusable lab equipment and consumables to meet the exacting needs of research and quality control laboratories.
Contact us today to find the perfect filtration solution for your application and experience the KINTEK difference in quality and performance.
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- High Purity Pure Graphite Crucible for Evaporation
People Also Ask
- Why are alumina crucibles selected for LTPO synthesis? Ensure Chemical Purity in High-Temperature Calcination
- Why are Platinum/Gold (Pt/Au) crucibles selected for silver phosphate glass? Ensure Maximum Purity in Glass Synthesis
- Why must alumina crucibles be configured inside static experimental tanks? Ensure Accuracy in Liquid Lead Tests
- What are the two types of crucibles and their uses? Choose the Right Crucible for Your Application
- What are the different types of crucible? A Guide to Material, Shape, and Application
- What are the advantages of using alumina crucibles for the TGA of modified alkyd resins? Ensure Accurate Results
- Why are magnesium oxide (MgO) crucibles utilized instead of standard metal crucibles? Ensure High-Purity Synthesis
- What makes a good crucible? Choose the Right Crucible for Your Metal Melting Needs



















