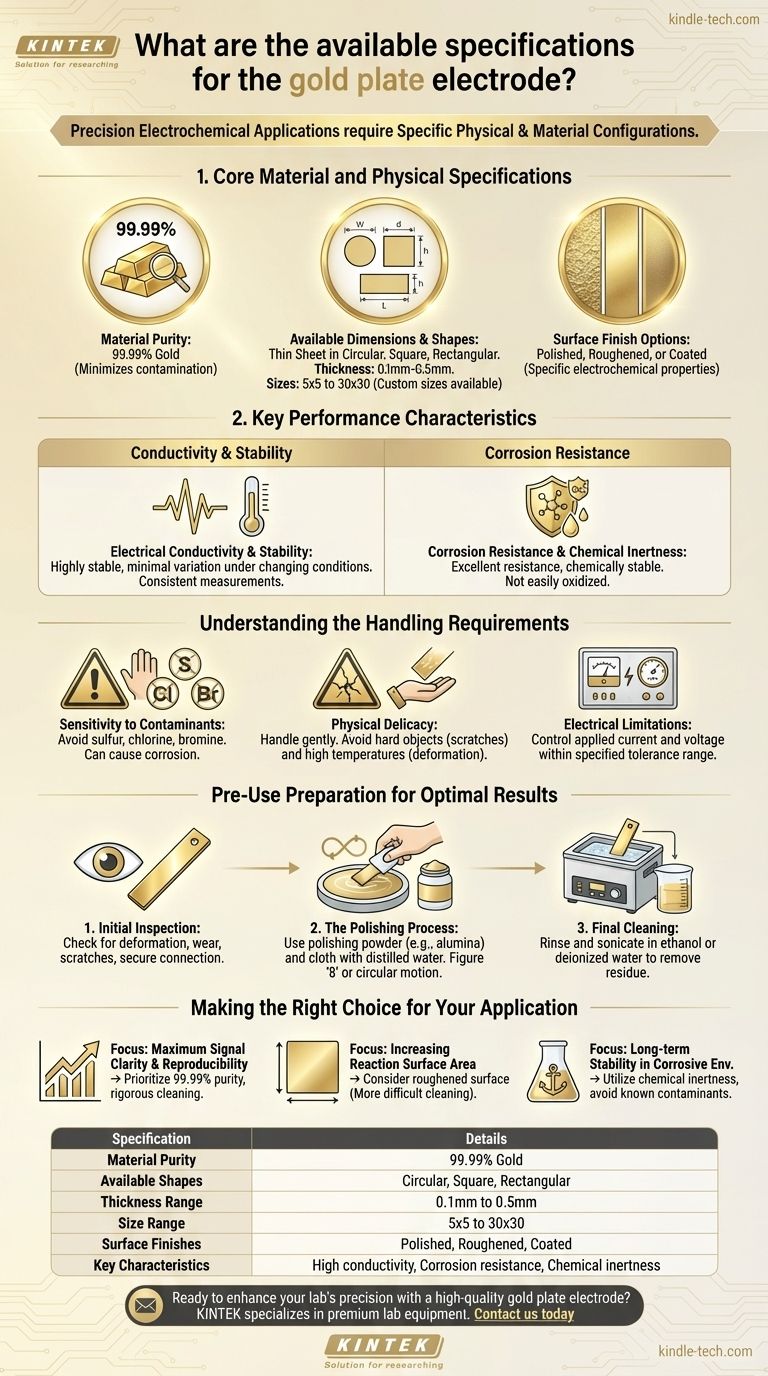
The gold plate electrode is available in several specific physical and material configurations. It is offered as a thin sheet in circular, square, or rectangular shapes, with a standard thickness from 0.1mm to 0.5mm and sizes from 5x5 to 30x30. Its material purity is 99.99%, and its surface can be polished, roughened, or coated to meet the demands of various applications.
The specifications of a gold plate electrode are not merely a list of dimensions; they are a direct reflection of its intended function in high-precision electrochemical applications. Understanding these properties, from purity to surface finish, is critical for ensuring experimental accuracy and reliability.

Core Material and Physical Specifications
The fundamental properties of the electrode dictate its performance. These specifications are the starting point for selecting the right tool for your experiment.
Material Purity (99.99%)
The electrode is made from gold with a purity of 99.99%. This high level of purity is essential for minimizing interference from contaminants, which ensures the accuracy and reliability of experimental data.
Available Dimensions and Shapes
Flexibility in form factor allows for adaptation to various cell geometries.
The electrode is produced as a thin sheet in circular, square, or rectangular shapes. Standard thickness ranges from 0.1mm to 0.5mm, with common sizes between 5x5 and 30x30. Custom sizes are also available upon request.
Surface Finish Options
The electrode surface can be modified to suit specific experimental needs. Standard options include polished, roughened, or coated surfaces, each offering different electrochemical properties, such as surface area or specific catalytic activity.
Key Performance Characteristics
The material choice of gold provides distinct advantages in electrochemical environments. These characteristics are why gold is a preferred material for many sensitive applications.
Electrical Conductivity and Stability
A gold plate electrode provides highly stable electrical conductivity. Its performance shows minimal variation even under changing temperatures and environmental conditions, leading to more consistent and repeatable measurements.
Corrosion Resistance and Chemical Inertness
Gold possesses excellent corrosion resistance and chemical stability. It is not easily oxidized or degraded in most electrochemical environments, ensuring the electrode maintains its structural integrity and performance over long-term use.
Understanding the Handling Requirements
While robust in its chemical properties, a gold electrode is a precision instrument that requires careful handling to maintain its performance and longevity.
Sensitivity to Contaminants
The electrode's surface is highly sensitive to specific elements. It is critical to keep the electrode away from substances containing sulfur, chlorine, and bromine, as these can cause corrosion and permanently alter the surface properties.
Physical Delicacy
The electrode is physically delicate and must be handled gently. Contact with hard objects can cause surface abrasion or scratches, which can compromise measurement accuracy. Likewise, exposure to excessively high temperatures should be avoided to prevent deformation.
Electrical Limitations
To prevent damage, you must control the applied current and voltage to stay within the electrode's specified tolerance range. Exceeding these limits can degrade the electrode.
Pre-Use Preparation for Optimal Results
Proper pretreatment is not optional; it is a mandatory step for achieving accurate and reproducible data.
Initial Inspection
Before every use, visually inspect the electrode for any signs of deformation, wear, or scratches. Ensure the connecting wire is secure.
The Polishing Process
To create a clean, uniform surface, polish the electrode using a specific procedure. Place a small amount of polishing powder (e.g., 1.0, 0.5, 0.3, down to 0.05 UM alumina) on a polishing cloth moistened with distilled water.
Hold the electrode vertically against the pad and polish in a figure '8' or circular motion. This ensures an even and smooth surface.
Final Cleaning
After polishing, the surface must be thoroughly cleaned. Rinse and sonicate the electrode in ethanol or deionized water to remove any residual polishing media and contaminants.
Making the Right Choice for Your Application
Your experimental goal should guide your choice of specification and handling protocol.
- If your primary focus is maximum signal clarity and reproducibility: Prioritize the 99.99% purity and follow a rigorous polishing and cleaning protocol before each use.
- If your primary focus is increasing reaction surface area: Consider a roughened surface finish, but be aware of the increased difficulty in cleaning and the potential for analyte adsorption.
- If your primary focus is long-term stability in a corrosive environment: The inherent chemical inertness of gold is your key advantage, but you must strictly avoid known contaminants like sulfur and chlorine.
Ultimately, treating your gold electrode as a precision instrument is the key to unlocking its full potential for reliable and accurate measurements.
Summary Table:
| Specification | Details |
|---|---|
| Material Purity | 99.99% Gold |
| Available Shapes | Circular, Square, Rectangular |
| Thickness Range | 0.1mm to 0.5mm |
| Size Range | 5x5 to 30x30 |
| Surface Finishes | Polished, Roughened, Coated |
| Key Characteristics | High conductivity, Corrosion resistance, Chemical inertness |
Ready to enhance your lab's precision with a high-quality gold plate electrode?
KINTEK specializes in premium lab equipment and consumables, offering reliable gold electrodes tailored to your specific electrochemical needs. Our 99.99% pure gold electrodes ensure accurate, reproducible results for sensitive applications.
Contact us today to discuss your requirements and let our experts help you select the perfect electrode for your research!
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What is the material and purity of a gold disc electrode? Ensuring Precision in Electrochemical Analysis
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan
- How should a gold disc electrode be maintained for long-term use? A Guide to Consistent Performance
- How should a gold disc electrode be handled during an experiment? Ensure Accurate Electrochemical Measurements
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data



















