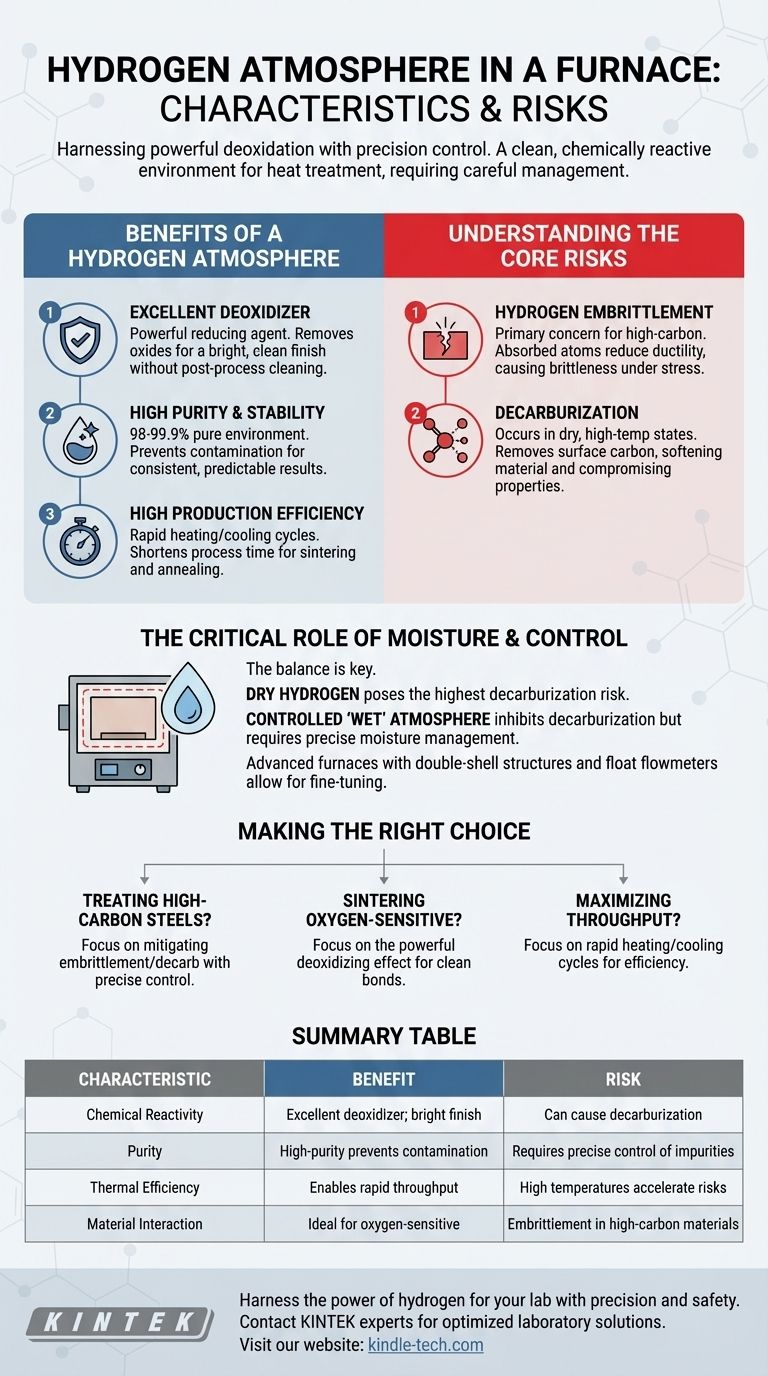
At its core, a hydrogen atmosphere in a furnace provides a highly pure, chemically reactive environment for heat treatment. Its primary characteristics are its exceptional ability to remove oxides and enable rapid processing, while its main risks involve altering the material's fundamental chemistry through decarburization and causing structural weakness via hydrogen embrittlement.
The central challenge of using a hydrogen atmosphere is harnessing its powerful deoxidizing properties without allowing its reactive nature to damage the material being treated. Success depends entirely on precise control over temperature and moisture content.

The Benefits of a Hydrogen Atmosphere
A hydrogen atmosphere is chosen for specific, high-stakes applications where a clean, controlled environment is paramount.
An Excellent Deoxidizer
Hydrogen is a powerful reducing agent, meaning it actively seeks out and reacts with oxygen.
This makes it extremely effective at cleaning the surface of materials by removing oxides, resulting in a bright, clean finish without the need for post-process cleaning.
High Purity and Stability
Furnaces designed for this work can provide a high-purity hydrogen environment, typically using commercial hydrogen that is 98% to 99.9% pure.
This ensures the material is not contaminated by other gases during heat treatment, leading to highly consistent and predictable results.
High Production Efficiency
Hydrogen's thermal properties, combined with specialized furnace design, allow for rapid heating and cooling cycles.
This significantly shortens the time required for high-temperature processes like sintering or annealing, directly improving production efficiency.
Understanding the Core Risks
The same chemical reactivity that makes hydrogen beneficial also introduces significant risks to the material itself if not managed carefully.
Hydrogen Embrittlement
This is a primary concern, especially for high-carbon substances.
Hydrogen atoms are small enough to be absorbed into the metal's internal structure. This absorption creates internal stress and reduces ductility, making the material brittle and prone to cracking under stress.
Decarburization
In a dry state and at high temperatures, hydrogen can react with the carbon within a material (like steel) to form methane (CH4) gas.
This process, known as decarburization, removes carbon from the material's surface, which can soften it and compromise its designed mechanical properties, such as hardness and strength.
The Critical Role of Moisture and Control
The effectiveness and risks of a hydrogen atmosphere are not static; they are directly influenced by the presence of impurities, most notably water vapor.
The Dry vs. Wet Hydrogen Paradox
A dry hydrogen atmosphere poses the highest risk of decarburization.
Conversely, intentionally introducing a controlled amount of moisture (a "wet" atmosphere) can inhibit decarburization. However, the moisture content itself becomes a critical process variable that must be precisely controlled.
Furnace Design and Control Systems
Hydrogen furnaces are specialized pieces of equipment designed to manage these variables.
They often feature a double-shell structure with advanced cooling to manage high temperatures and maintain pressure integrity.
Crucially, they include systems like float flowmeters for precise control over the flow of hydrogen and other gases (like nitrogen for purging), allowing operators to fine-tune the atmospheric conditions.
Making the Right Choice for Your Process
Applying a hydrogen atmosphere requires a clear understanding of your material and your primary processing goal.
- If your primary focus is treating high-carbon steels: Your main challenge is mitigating hydrogen embrittlement and decarburization, which demands precise control over temperature and moisture levels.
- If your primary focus is sintering oxygen-sensitive materials: The powerful deoxidizing effect is your greatest asset, ensuring a clean, bright finish and strong metallurgical bonds.
- If your primary focus is maximizing throughput: The rapid heating and cooling cycles enabled by hydrogen can significantly boost your production efficiency.
Ultimately, a hydrogen atmosphere offers unparalleled performance for specific applications but demands a disciplined and knowledgeable approach to control its inherent risks.
Summary Table:
| Characteristic | Benefit | Risk |
|---|---|---|
| Chemical Reactivity | Excellent deoxidizer; removes oxides for a bright finish | Can cause decarburization, softening the material |
| Purity | High-purity (98-99.9%) environment prevents contamination | Requires precise control of impurities like moisture |
| Thermal Efficiency | Enables rapid heating/cooling for high production throughput | High temperatures accelerate risks like hydrogen embrittlement |
| Material Interaction | Ideal for sintering oxygen-sensitive materials | Hydrogen embrittlement can make high-carbon materials brittle |
Harness the power of hydrogen for your lab with precision and safety.
At KINTEK, we specialize in advanced laboratory furnaces and consumables designed for controlled atmosphere applications. Whether you are sintering sensitive materials or heat-treating high-carbon steels, our expertise ensures you achieve superior results while mitigating risks like embrittlement and decarburization.
Let us help you optimize your process for efficiency and material integrity. Contact our experts today to discuss your specific laboratory needs and discover the right equipment solution for you.
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the use of hydrogen in annealing? Purify Metals and Prevent Brittleness
- What is a retort furnace for heat treating? Achieve Superior Atmospheric Control for Your Materials
- What are the typical gas compositions for nitrogen-based atmospheres? Expert Guide to Thermal Processing Ratios
- What is a chemically reducing atmosphere? A Guide to Oxidation-Free Environments
- Why is nitrogen gas used in annealing process? Prevent Oxidation and Achieve Superior Metal Properties
- What is an inert gas and which processes is it used in? A Guide to Protective Atmospheres
- What role does a high-temperature annealing furnace play in the formation of ohmic contacts for diamond devices?
- How does the temperature control precision of a solid-state reaction sintering furnace affect lithium-rich materials?



















