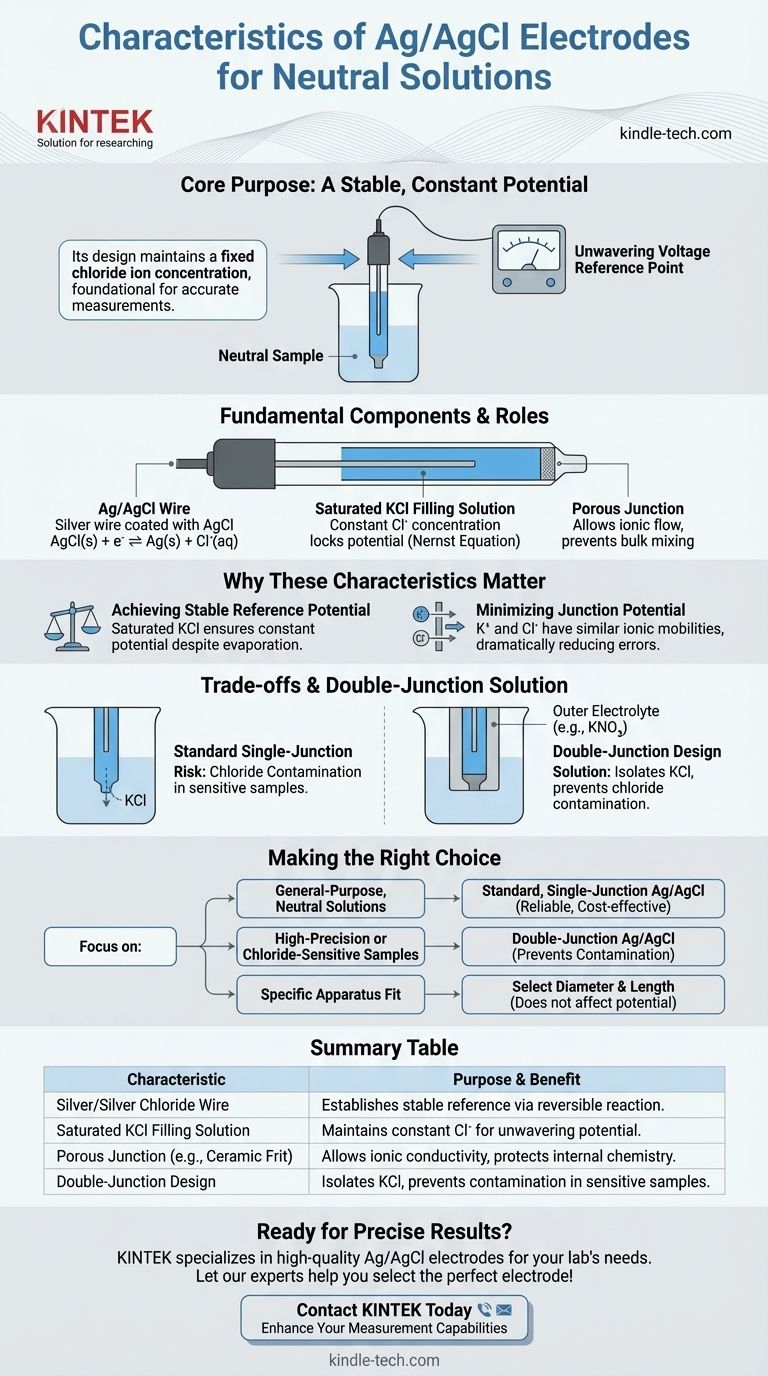
At its core, a silver/silver chloride (Ag/AgCl) electrode designed for neutral solutions is a reference electrode that provides a stable, constant potential. Its key characteristic is the use of a silver wire coated in silver chloride, which is immersed in a saturated solution of potassium chloride (KCl) to maintain a fixed chloride ion concentration.
The purpose of an Ag/AgCl electrode is not to react with your sample, but to provide an unwavering, known voltage reference point. Its design, centered on a saturated KCl solution, ensures this stability, which is the foundation for any accurate electrochemical measurement.

The Fundamental Components and Their Role
To understand the electrode's performance, you must first understand its construction. Each part serves a precise function to guarantee a stable potential.
The Silver/Silver Chloride Element
The heart of the electrode is a pure silver wire coated with a layer of silver chloride (AgCl). This element is where the fundamental electrochemical reaction occurs.
The potential is established by the equilibrium between the solid silver/silver chloride and the chloride ions (Cl⁻) in the filling solution: AgCl(s) + e⁻ ⇌ Ag(s) + Cl⁻(aq).
The Saturated KCl Filling Solution
For use in neutral solutions, the electrode is filled with a saturated potassium chloride (KCl) solution. This is the most critical feature for stability.
Because the solution is saturated, the concentration of chloride ions is held constant at a known, high level. According to the Nernst equation, this constant chloride concentration locks the electrode's potential, making it a reliable reference.
The Porous Junction
A porous frit, typically made of ceramic or quartz, separates the internal filling solution from the external sample solution.
This junction allows ions to flow between the electrode and the sample, completing the electrical circuit. However, it prevents the bulk mixing of the two solutions, protecting the internal chemistry of the reference electrode.
Why These Characteristics Matter for Your Measurement
The specific components of an Ag/AgCl electrode are deliberately chosen to solve common problems in electrochemistry, ensuring your results are accurate and reproducible.
Achieving a Stable Reference Potential
The entire purpose of a reference electrode is to have a potential that does not change during your experiment.
By using a saturated KCl solution, the chloride concentration remains constant even if a small amount of water evaporates or diffuses. This constant concentration ensures a rock-solid reference potential against which you can measure your working electrode.
Minimizing the Junction Potential
A major source of error in electrochemical measurements is the liquid junction potential. This is a small voltage that develops at the interface between the filling solution and your sample solution due to differences in ionic diffusion rates.
Potassium chloride (KCl) is used specifically because the ionic mobilities of the potassium ion (K⁺) and chloride ion (Cl⁻) are nearly identical. This similarity causes them to diffuse across the junction at almost the same rate, which dramatically minimizes the junction potential and improves measurement accuracy.
Understanding the Trade-offs and Variations
While the standard Ag/AgCl electrode is a workhorse, it's not universally perfect. Understanding its limitations is key to avoiding measurement errors.
The Risk of Chloride Contamination
The primary trade-off is that a small amount of the KCl filling solution will inevitably leak from the porous junction into your sample.
For most neutral solutions, this is not a problem. However, if your experiment is sensitive to chloride ions—for example, if you are measuring low concentrations of chloride or working with samples that precipitate with chloride (like silver nitrate)—this leakage will introduce significant errors.
The Role of the Double-Junction Electrode
To solve the problem of chloride contamination, a double-junction electrode is used. This design features a second outer chamber that isolates the KCl solution from the sample.
This outer chamber can be filled with a non-interfering electrolyte, such as potassium nitrate (KNO₃) or sodium sulfate (Na₂SO₄). It provides the ionic bridge to the sample without introducing chloride ions, preserving your sample's integrity.
Physical Design Considerations
Electrodes are available in various diameters (e.g., 4mm, 6mm, 10mm) and lengths. These physical characteristics do not affect the electrode's potential but are chosen based on the constraints of your electrochemical cell, the volume of your sample, and the required durability.
Making the Right Choice for Your Goal
Selecting the correct electrode is a critical step for acquiring reliable data. Your choice depends directly on the chemical nature of your sample.
- If your primary focus is general-purpose measurements in neutral solutions: A standard, single-junction Ag/AgCl electrode with saturated KCl is the reliable and cost-effective choice.
- If your primary focus is high-precision measurements or chloride-sensitive samples: You must use a double-junction Ag/AgCl electrode to prevent sample contamination and ensure data accuracy.
- If your primary focus is fitting into a specific apparatus: Select the electrode diameter and length that physically matches the design of your electrochemical cell.
Understanding how these components create a stable reference is the key to generating trustworthy and reproducible electrochemical results.
Summary Table:
| Characteristic | Purpose & Benefit |
|---|---|
| Silver/Silver Chloride Wire | Core element; establishes a stable reference potential via a reversible reaction. |
| Saturated KCl Filling Solution | Maintains constant chloride ion concentration for unwavering potential, even with minor evaporation. |
| Porous Junction (e.g., Ceramic Frit) | Allows ionic conductivity while preventing bulk mixing of solutions, protecting internal chemistry. |
| Double-Junction Design (Variation) | Isolates KCl from sample using an intermediate electrolyte to prevent chloride contamination in sensitive samples. |
Ready to achieve precise and reliable electrochemical results?
The right reference electrode is fundamental to your lab's success. KINTEK specializes in high-quality lab equipment, including a range of Ag/AgCl electrodes designed for accuracy and durability in neutral solutions. Whether you need a standard single-junction model for general use or a double-junction electrode for chloride-sensitive applications, we have the solution to meet your specific laboratory needs.
Let our experts help you select the perfect electrode for your experiments. Contact KINTEK today to discuss your requirements and enhance your measurement capabilities!
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What are the characteristics of a saturated calomel electrode for neutral solutions? Understanding its stability and limitations.
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- What are the general precautions for using a reference electrode? Ensure Stable Potentials for Accurate Data
- Why and how should the electrodes of an electrolytic cell be calibrated? Ensure Reliable Results
- What is the reference electrode for mercury mercury chloride? Discover the Saturated Calomel Electrode (SCE)



















