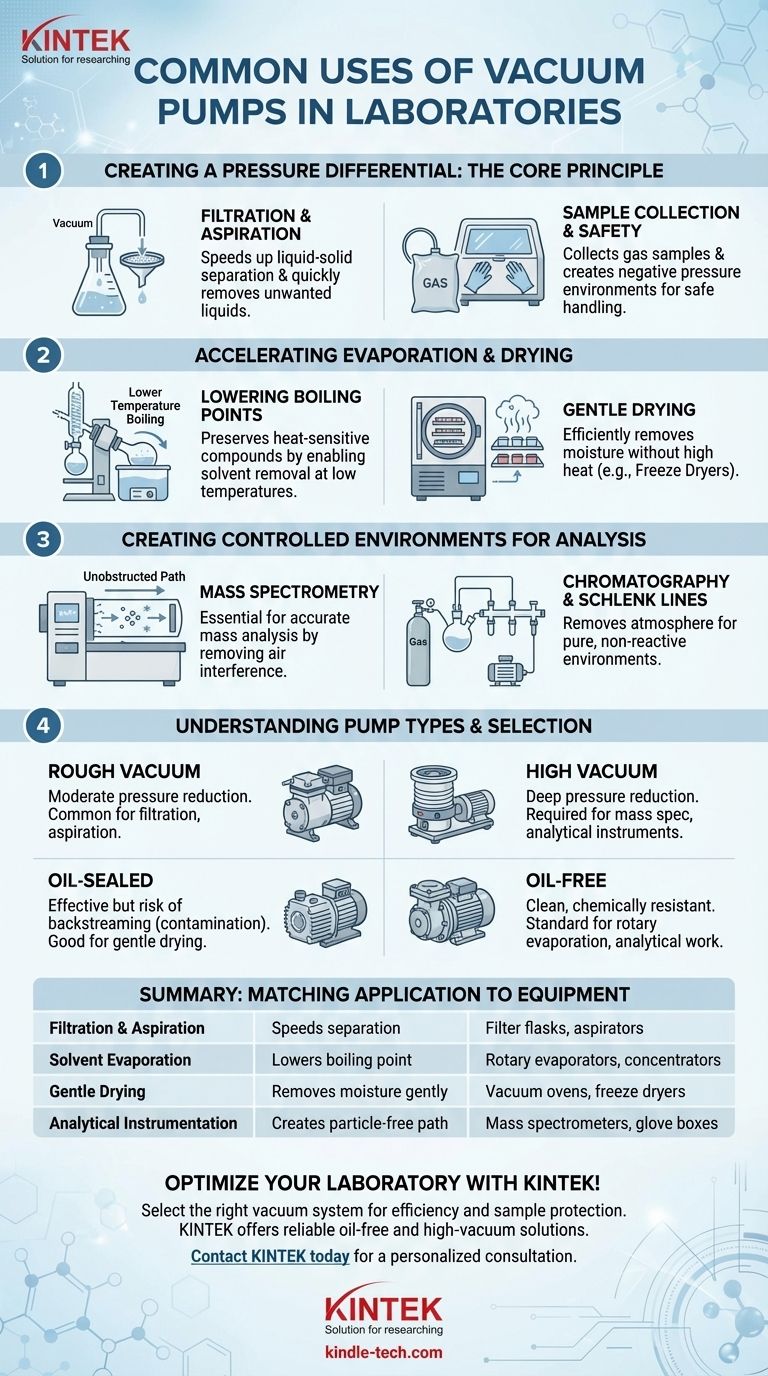
In the modern laboratory, vacuum pumps are indispensable tools used for a wide range of critical tasks. Their most common applications include filtering liquids, aspirating waste fluids, accelerating the evaporation of solvents in rotary evaporators and concentrators, and creating the controlled, low-pressure environments required for sensitive analytical instruments like mass spectrometers.
At its core, a vacuum pump is more than a simple suction device. It is a tool for manipulating pressure to achieve a specific physical or chemical goal, whether that's speeding up a physical process, protecting a sensitive sample, or enabling a precise measurement.

The Core Principle: Creating a Pressure Differential
A vacuum pump works by removing gas molecules from a sealed system, creating a pressure lower than the surrounding atmosphere. This pressure difference is the driving force behind several fundamental lab applications.
For Filtration and Aspiration
Filtration under gravity can be a slow process. By connecting a vacuum pump to a filter flask, you create negative pressure beneath the filter paper.
This pressure differential actively pulls the liquid through the filter medium, dramatically speeding up the separation of a solid from a liquid. A similar principle applies to aspiration, where a vacuum is used to quickly and safely remove unwanted liquids from a container.
For Sample Collection and Safety
A vacuum can be used to collect gas samples from a test chamber or even the atmosphere, pulling the gas into a collection vessel.
Furthermore, working with hazardous materials inside a vacuum-sealed glove box or fume hood creates an environment of negative pressure. If a small leak were to occur, ambient air would flow into the chamber rather than hazardous fumes flowing out, providing a critical layer of safety.
Accelerating Evaporation and Drying
One of the most powerful uses of a vacuum is its ability to alter the physical properties of liquids, specifically their boiling points. This is essential for gently processing heat-sensitive compounds.
Lowering the Boiling Point of Solvents
A liquid boils when its vapor pressure equals the pressure of the environment around it. By reducing the ambient pressure with a vacuum pump, you significantly lower the temperature at which a solvent will boil.
This principle is the cornerstone of equipment like the rotary evaporator and solvent concentrator. It allows for the rapid removal of solvents at low temperatures, preserving the integrity of thermally sensitive compounds that would otherwise decompose.
Gentle Drying of Sensitive Samples
Equipment like vacuum ovens, desiccators, and gel dryers also leverage this principle. Placing a sample under vacuum removes residual water or solvents far more efficiently and gently than heat alone.
Freeze dryers (lyophilizers) take this to an extreme, using a deep vacuum to turn frozen water directly into vapor (sublimation), which is the gentlest possible way to produce a stable, dry sample.
Creating Controlled Environments for Analysis
Many advanced analytical techniques are only possible in a near-perfect vacuum, where atmospheric gases would otherwise interfere with the measurement.
Mass Spectrometry
In a mass spectrometer, ions must travel from their source to a detector without colliding with other molecules. Even a tiny amount of air would cause constant collisions, scattering the ions and making detection impossible.
A high-vacuum system ensures a clear, unobstructed path, which is absolutely critical for achieving accurate mass analysis.
Chromatography and Schlenk Lines
In certain types of gas chromatography and in air-sensitive chemistry using a Schlenk line, a vacuum is used to completely remove the atmosphere from the system. The system can then be backfilled with an inert gas, like nitrogen or argon.
This process ensures that the experiment or analysis is conducted in a perfectly pure and non-reactive environment, preventing unwanted side reactions or contamination.
Understanding the Trade-offs: Not All Vacuums Are Equal
The term "vacuum" covers a wide range of pressures, and choosing the right pump is critical for success. The wrong choice can lead to inefficient processes or even damaged samples.
Vacuum Level: Rough vs. High Vacuum
Simple applications like filtration only require a rough vacuum, which is a moderate reduction in pressure. This can be achieved with a simple, robust pump.
Conversely, instruments like mass spectrometers require a high vacuum—a pressure many orders ofmagnitude lower. This often requires a multi-stage system with both a primary "roughing" pump and a secondary "high-vacuum" pump (like a turbomolecular or diffusion pump).
Pump Type: Oil-Sealed vs. Oil-Free
Traditional rotary vane pumps use oil to create a seal and lubricate parts. While effective, they carry a risk of "backstreaming," where oil vapor can contaminate the vacuum system and ruin sensitive samples.
Modern oil-free diaphragm pumps have become the standard for most lab applications like rotary evaporation. They provide a clean, chemically resistant vacuum without the risk of oil contamination, making them ideal for analytical and chemical work.
Matching the Pump to the Application
To ensure efficiency and protect your work, select a vacuum system based on your specific goal.
- If your primary focus is sample preparation (filtration, aspiration): A basic, water-jet aspirator or a simple, robust diaphragm pump will be sufficient and cost-effective.
- If your primary focus is solvent removal (rotary evaporation, concentration): A chemically resistant, oil-free diaphragm pump with good vacuum control is the industry standard.
- If your primary focus is gentle drying (freeze-drying, vacuum ovens): You need a system capable of deeper vacuum levels, often a two-stage diaphragm pump or an oil-sealed rotary vane pump.
- If your primary focus is high-sensitivity analysis (mass spectrometry): You require a dedicated high-vacuum system, typically involving a combination of roughing and turbomolecular pumps.
Understanding the principle behind the application is the key to selecting the right tool for the job.
Summary Table:
| Application | Key Function | Common Equipment |
|---|---|---|
| Filtration & Aspiration | Speeds up liquid-solid separation | Filter flasks, aspirators |
| Solvent Evaporation | Lowers boiling point for gentle removal | Rotary evaporators, concentrators |
| Gentle Drying | Removes moisture without high heat | Vacuum ovens, freeze dryers |
| Analytical Instrumentation | Creates particle-free path for analysis | Mass spectrometers, glove boxes |
Optimize your laboratory processes with the right vacuum pump from KINTEK!
Whether your work involves routine filtration, sensitive sample preparation, or high-precision analytical instrumentation, selecting the correct vacuum system is critical for efficiency and protecting your samples. KINTEK specializes in providing reliable lab equipment and consumables, including a range of oil-free diaphragm pumps and high-vacuum systems tailored to your specific application—from chemistry and biology to materials science.
Let our experts help you enhance your lab's capabilities. Contact KINTEK today for a personalized consultation and discover the vacuum solution that will drive your research forward.
Visual Guide
Related Products
- Laboratory Rotary Vane Vacuum Pump for Lab Use
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Variable Speed Peristaltic Pump
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
People Also Ask
- What is the specific function of quartz glass sample holders? Optimize Kinetic Data in High-Temp Corrosion Studies
- What role does a laboratory cold trap play in high-temperature corrosion experiments? Mastering Phase Control
- Why is precise temperature program control necessary for carbonization furnaces? Unlock Charcoal Quality and Efficiency
- What is the role of a laboratory heating system in electrolyte ohmic resistance? Optimize Precise Thermal Analysis
- What are the parts that go to a furnace? A Guide to the Three Core Systems
- How does the performance of single-stage and two-stage rotary vane pumps compare? Optimize Your Vacuum Efficiency
- What is the function of a magnetic stirrer in SiOC film preparation? Ensure Precision in Precursor Mixing
- Why are zirconia grinding balls preferred for NiCrAlY-Mo-Ag powders? Ensure Maximum Purity and Durability



















