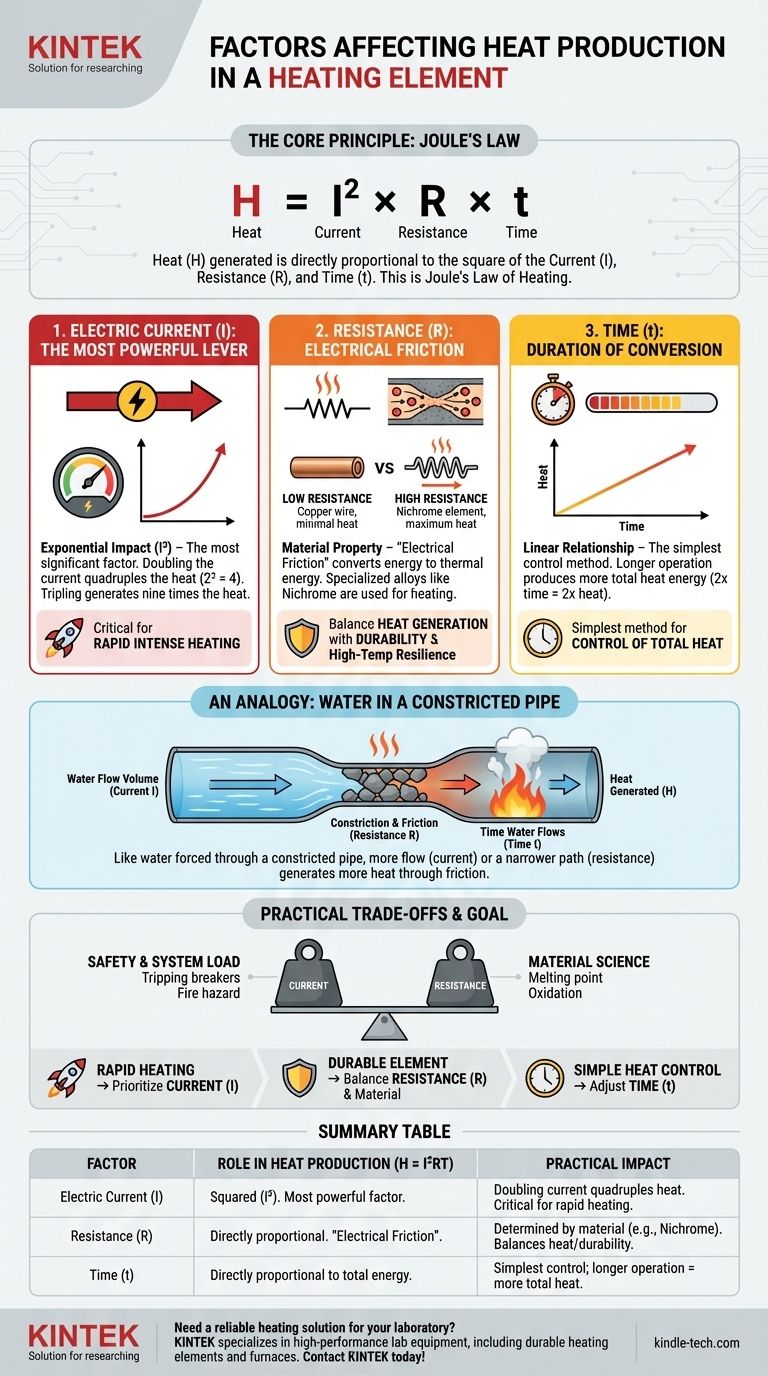
At its core, the heat produced in a heating element is determined by three fundamental factors. These are the amount of electric current flowing through the element, the electrical resistance of the element's material, and the time for which the current flows.
The amount of heat generated is not simply a list of influences; it is governed by a precise physical principle called Joule's Law of Heating. This law states that heat is directly proportional to the square of the current, the resistance, and the time, giving you specific levers to control the outcome.

The Core Principle: Joule's Law
The relationship between electricity and heat in a conductor was quantified by James Prescott Joule in the 19th century. This principle is the foundation for everything from electric stoves to industrial furnaces.
The Governing Formula: H = I²RT
The heat (H) generated is the product of the square of the current (I²), multiplied by the resistance (R), and the time (t) the current flows.
This formula, H = I²RT, is the key to understanding and controlling resistive heating. Each variable plays a distinct and critical role in the final heat output.
An Analogy: Water in a Constricted Pipe
Imagine electricity as water flowing through a pipe. The current (I) is the volume of water flowing per second. The resistance (R) is like a narrow, constricted section of that pipe filled with gravel.
As water is forced through this constricted section, friction generates heat. The more water you force through (higher current) or the narrower and rougher the constriction (higher resistance), the more heat is produced.
Deconstructing the Factors
To effectively design or troubleshoot a heating system, you must understand the unique impact of each variable in Joule's equation.
Electric Current (I): The Most Powerful Lever
The most significant factor in the equation is the current. Because it is squared (I²), its impact on heat production is exponential.
If you double the current, you quadruple the heat produced. If you triple the current, you generate nine times the heat. This makes current adjustment the most powerful method for increasing heat output.
Resistance (R): The Source of "Electrical Friction"
Resistance is an intrinsic property of a material that impedes the flow of electrons. This "electrical friction" is what converts electrical energy into thermal energy.
Materials like copper have very low resistance and are used for wires to minimize heat loss. Conversely, heating elements are made from materials with high resistance, such as Nichrome (a nickel-chromium alloy), to maximize heat generation.
Time (t): The Duration of Energy Conversion
This is the most straightforward factor. Heat is a measure of energy, so the longer you apply power to the element, the more total heat will be generated.
The relationship is linear: if you run the element for twice as long, you will produce twice the total amount of heat, assuming current and resistance remain constant.
Understanding the Practical Trade-offs
While the formula seems simple, real-world application involves balancing these factors against material limitations and safety.
Current vs. Safety and System Load
Drastically increasing current is not always feasible. It requires thicker, more expensive wiring to handle the load and can trip circuit breakers or create a fire hazard if not managed properly.
Resistance vs. Material Science
A material with very high resistance is ideal for generating heat, but it must also have a high melting point and resist oxidation at extreme temperatures. This is why specialized alloys are necessary; a simple iron wire would quickly degrade and fail.
The Interplay with Voltage (Ohm's Law)
In most practical applications (like a wall outlet), you are supplied with a constant voltage (V), not a constant current. According to Ohm's Law (V = IR), voltage, current, and resistance are linked.
This means if you change the resistance of the heating element (R) in a constant-voltage system, you will also change the current (I). An element with lower resistance will draw more current and, due to the I² term, can actually produce more heat in a fixed-voltage circuit.
Making the Right Choice for Your Goal
Your primary objective dictates which factor you should prioritize for optimization.
- If your primary focus is rapid, intense heating: Prioritize increasing the current (I), as its squared effect provides the greatest impact on power output.
- If your primary focus is designing a durable element: The key is selecting a material with the optimal balance of high resistance (R) and high-temperature resilience.
- If your primary focus is simple control of total heat: Adjusting the time (t) the element is powered on is the most direct and easily managed method.
Ultimately, mastering heat production is about understanding and applying the precise and predictable principles of Joule's Law.
Summary Table:
| Factor | Role in Heat Production (H = I²RT) | Practical Impact |
|---|---|---|
| Electric Current (I) | Squared in the formula (I²). The most powerful factor. | Doubling the current quadruples the heat. Critical for rapid heating. |
| Resistance (R) | Directly proportional to heat. The source of "electrical friction." | Determined by element material (e.g., Nichrome). Balances heat generation with durability. |
| Time (t) | Directly proportional to total heat energy. | The simplest control method; longer operation = more total heat. |
Need a reliable heating solution for your laboratory?
Understanding the principles of Joule's Law is the first step; applying them with the right equipment is the next. KINTEK specializes in high-performance lab equipment, including durable heating elements and furnaces designed for precise temperature control and long-lasting performance.
Let our experts help you select the ideal heating system for your specific application. Contact KINTEL today to discuss your laboratory's heating needs!
Visual Guide
Related Products
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Rotating Platinum Disk Electrode for Electrochemical Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What is the thermal expansion coefficient of molybdenum disilicide? Understanding its role in high-temperature design
- What is molybdenum disilicide used for? Powering High-Temperature Furnaces Up to 1800°C
- Which high temperature furnace elements to be used in oxidizing atmosphere? MoSi2 or SiC for Superior Performance
- What function do molybdenum disilicide heating elements perform? Precision Heat for Pulverized Coal Research
- What are the properties of molybdenum heating element? Choose the Right Type for Your Furnace Atmosphere
















