In controlled furnace atmospheres, nitrogen (N2) primarily serves two key functions: acting as a passive, inert carrier gas and providing a protective atmosphere to prevent unwanted chemical reactions. It is used to displace reactive gases like oxygen and water vapor, preventing oxidation, and can be tailored for specific processes like annealing low-carbon steels or the general heat treatment of high-carbon steels.
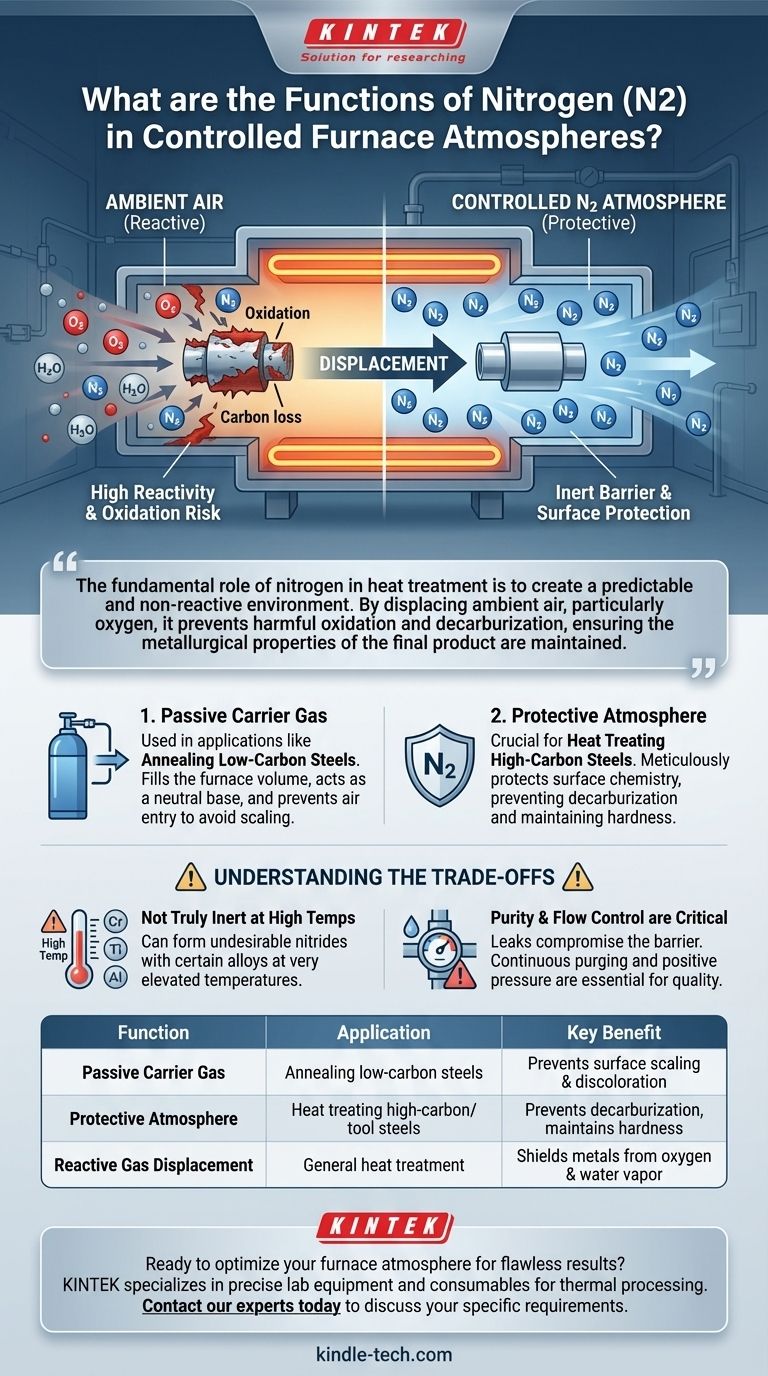
The fundamental role of nitrogen in heat treatment is to create a predictable and non-reactive environment. By displacing ambient air, particularly oxygen, it prevents harmful oxidation and decarburization, ensuring the metallurgical properties of the final product are maintained.

The Primary Role: Displacing Reactive Gases
The air around us, composed of roughly 79% nitrogen and 21% oxygen, is highly reactive at the elevated temperatures found in a furnace. Introducing a controlled nitrogen atmosphere is fundamentally about removing these reactive elements.
Preventing Oxidation
Oxygen (O2) is the primary cause of oxidation, or scaling, on the surface of metals during heat treatment. This oxide layer can be detrimental to a part's final dimensions and surface finish.
By flooding the furnace chamber with nitrogen, you physically push out, or displace, the oxygen. This creates an environment where the metal surface is shielded from reacting with O2.
Avoiding Decarburization
For high-carbon steels, another risk is decarburization, which is the loss of carbon content from the surface of the steel. This process weakens the material and compromises its hardness.
Gases like oxygen, carbon dioxide, and water vapor can all strip carbon from steel at high temperatures. A nitrogen-rich atmosphere helps prevent this by providing a neutral backdrop that does not react with the carbon in the steel.
Nitrogen's Two Functional States
While the core purpose is protection, nitrogen is applied in slightly different ways depending on the metallurgical goal.
As a Passive Carrier Gas
In many applications, such as the annealing of low-carbon steels, nitrogen is used in a passive or "inert" state. Its job is simply to fill the furnace volume and prevent air from entering.
It can also act as a carrier gas, meaning it serves as a neutral base to which small, controlled amounts of active gases (like hydrogen or carbon monoxide) can be added for more complex processes.
As a Protective Atmosphere
When heat treating more sensitive materials like high-carbon steels, the term "protective atmosphere" is more appropriate. The goal is not just to prevent gross scaling but to meticulously protect the precise surface chemistry of the part.
In this context, high-purity nitrogen ensures that no unintended reactions occur, preserving the integrity and performance characteristics of the steel.
Understanding the Trade-offs
While incredibly useful, nitrogen is not a universal solution, and its application requires careful consideration.
Not Truly Inert at High Temperatures
Although often treated as inert, nitrogen can become reactive at very high temperatures with certain alloying elements. For example, it can form nitrides with elements like chromium, titanium, and aluminum, which can be undesirable in some applications.
Purity and Flow Control are Critical
The effectiveness of a nitrogen atmosphere depends entirely on its purity and the furnace's integrity. Any leaks that allow air to enter will compromise the protective barrier.
Proper gas flow control is essential to maintain positive pressure inside the furnace, continuously purging any contaminants and ensuring part quality.
Making the Right Choice for Your Process
Your choice of atmosphere depends directly on the material being treated and the desired metallurgical outcome.
- If your primary focus is simple annealing of low-carbon steel: Use nitrogen as a cost-effective, passive atmosphere to prevent surface scaling and discoloration.
- If your primary focus is heat treating high-carbon or tool steels: Use high-purity nitrogen as a protective base to prevent decarburization and maintain critical surface hardness.
- If your primary focus is treating highly reactive metals (e.g., titanium): Recognize that nitrogen can be reactive and may necessitate a true inert gas like argon or the use of a vacuum furnace.
Ultimately, using nitrogen effectively is about controlling the furnace environment to achieve precise, repeatable metallurgical results.
Summary Table:
| Function | Application | Key Benefit |
|---|---|---|
| Passive Carrier Gas | Annealing low-carbon steels | Prevents surface scaling & discoloration |
| Protective Atmosphere | Heat treating high-carbon/tool steels | Prevents decarburization, maintains hardness |
| Reactive Gas Displacement | General heat treatment | Shields metals from oxygen & water vapor |
Ready to optimize your furnace atmosphere for flawless results?
KINTEK specializes in providing the precise lab equipment and consumables you need to control your thermal processing environment. Whether you are annealing low-carbon steel or performing critical heat treatment on high-carbon alloys, our expertise ensures you achieve consistent, high-quality metallurgical outcomes.
Contact our experts today to discuss how we can support your laboratory's specific furnace atmosphere requirements.
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What are the typical air-to-gas ratios for endothermic generators? Optimize Natural Gas and Propane Settings
- What are the different types of brazing gas? Choose the Right Atmosphere for Strong, Clean Joints
- What is the necessity of integrating an analytical balance with an atmosphere furnace for TGA? Mastering Kinetic Data
- Why is a vacuum or atmosphere furnace required for SiBCN pyrolysis? Mastering Precision for Superior Ceramics
- What type of atmosphere must high-temperature atmosphere sintering furnaces provide? Optimizing Boron Carbide Sintering
- What is the purpose of using an atmosphere tube furnace for LLZTO coating? Enhance Solid-State Battery Performance
- How does an atmosphere heating furnace used for surface pre-oxidation assist in subsequent nitriding? Boost Surface Activity
- How is a reducing atmosphere used in foundry operations? Essential Guide to Iron Ore Reduction and Metal Refining



















