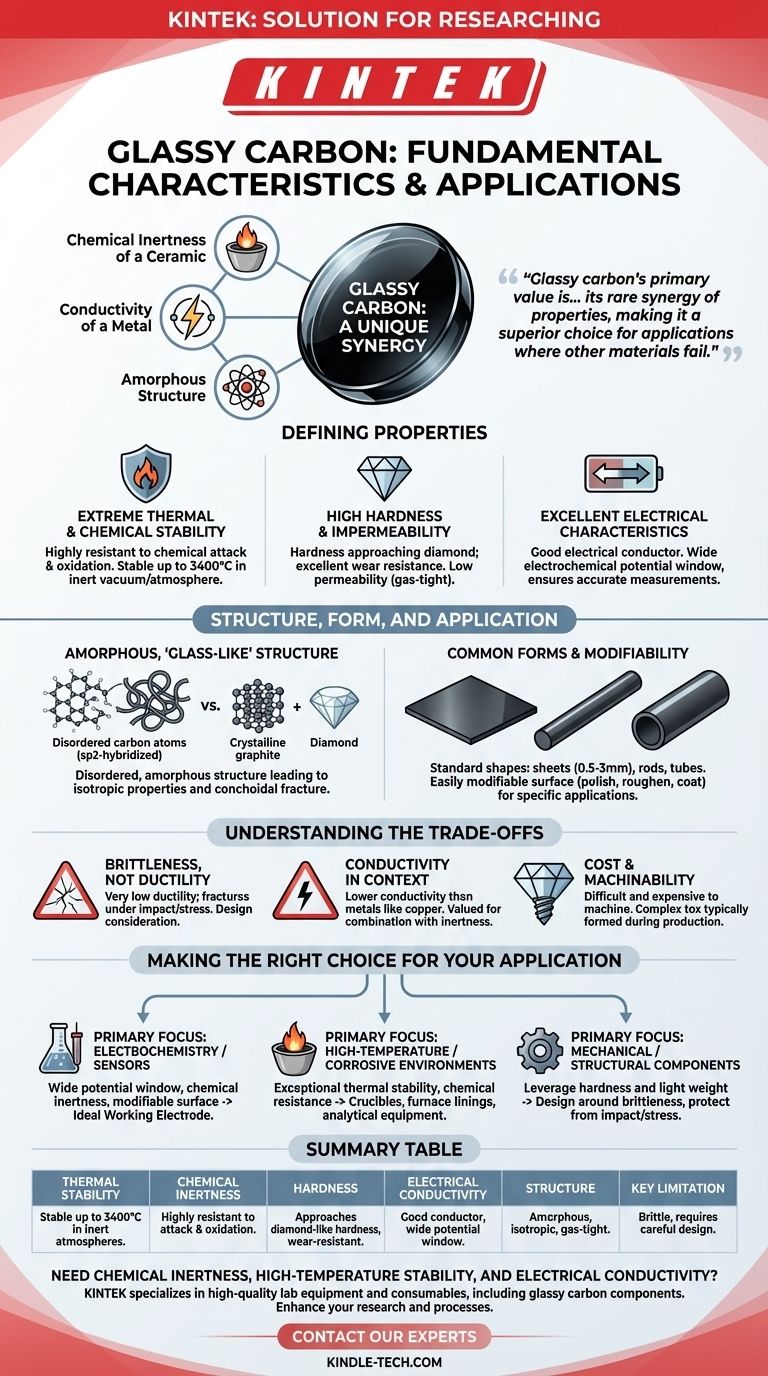
At its core, glassy carbon is a unique, non-graphitizing form of carbon that combines properties typically found in very different classes of materials. It is renowned for its exceptional resistance to high temperatures and chemical attack, high hardness approaching that of a diamond, and good electrical conductivity. This combination makes it a highly specialized and valuable material in advanced technical fields.
Glassy carbon's primary value is not in one single characteristic, but in its rare synergy of properties. It offers the chemical inertness of a ceramic, the conductivity of a metal, and a unique, disordered atomic structure, making it a superior choice for applications where other materials fail.

The Defining Properties of Glassy Carbon
Glassy carbon, also known as vitreous carbon, derives its name from its glass-like appearance and fracture behavior. Its performance is rooted in a unique set of physical and chemical characteristics.
Extreme Thermal and Chemical Stability
The most notable feature of glassy carbon is its resilience. It is highly resistant to chemical attack and oxidation, maintaining its integrity in environments that would degrade most other materials.
Furthermore, it can withstand extremely high temperatures, remaining stable up to 3400°C when in a vacuum or an inert atmosphere. This makes it suitable for high-temperature crucibles and furnace components.
High Hardness and Impermeability
Glassy carbon exhibits a hardness that approaches that of a diamond, giving it excellent resistance to wear and abrasion.
This hardness is coupled with very low permeability to both gases and liquids. This "gas-tight" nature is a direct result of its disordered, amorphous atomic structure, which lacks the voids and pathways present in more porous materials.
Excellent Electrical Characteristics
Unlike most ceramic-like materials, glassy carbon is a good electrical conductor. While not a direct replacement for copper in general wiring, its conductivity is more than sufficient for its most common applications.
Crucially for electrochemistry, it possesses a very wide potential window. This means it remains inert and does not react over a broad range of applied voltages, ensuring that the measurements reflect the chemical reaction of interest, not the electrode itself.
Structure, Form, and Application
The utility of glassy carbon is defined as much by its structure and available forms as by its intrinsic properties. Understanding this link is key to using it effectively.
An Amorphous, "Glass-Like" Structure
Unlike graphite (crystalline layers) or diamond (a rigid crystal lattice), glassy carbon has a disordered, amorphous structure. It consists of tangled ribbons of sp2-hybridized carbon atoms, similar to graphite, but without the long-range order.
This lack of a crystalline structure is responsible for its isotropic properties (uniform in all directions) and its conchoidal, glass-like fracture pattern. It also allows for forms like Reticulated Vitreous Carbon (RVC), which have an open-pore, foam-like structure with a very large surface area.
Common Forms and Modifiability
Glassy carbon is produced in standard industrial shapes, most commonly as flat sheets, rods, or tubes. Sheets are typically available in thicknesses from 0.5mm to 3mm.
A key advantage is that its surface is easily modifiable. It can be polished to a mirror finish, roughened to increase surface area, or coated with other materials to create highly specific and sensitive surfaces for sensors and electrochemical analysis.
Understanding the Trade-offs
No material is perfect. To use glassy carbon effectively, you must be aware of its limitations.
Brittleness, Not Ductility
Like other extremely hard materials such as ceramics, glassy carbon is brittle. It has very low ductility and will fracture under sharp impact or excessive bending stress rather than deforming. This must be a primary consideration in any mechanical design.
Conductivity in Context
While its electrical conductivity is good for a non-metal, it is significantly lower than that of metallic conductors like copper or gold. Its value comes from being conductive while also being chemically inert and hard, a combination that metals cannot offer.
Cost and Machinability
The same hardness that makes glassy carbon durable also makes it difficult and expensive to machine. Complex shapes are typically formed during the initial production process, as post-processing requires specialized diamond tooling.
Making the Right Choice for Your Application
Selecting glassy carbon should be a deliberate decision based on its unique strengths.
- If your primary focus is electrochemistry or sensors: Its wide potential window, chemical inertness, and modifiable surface make it the default choice for a reliable working electrode.
- If your primary focus is a high-temperature or corrosive environment: Its exceptional thermal stability and resistance to chemical attack are its most valuable assets for crucibles, furnace linings, or analytical equipment.
- If your primary focus is a mechanical or structural component: You must design around its brittleness, leveraging its hardness and light weight while protecting it from impact and flexural stress.
Ultimately, glassy carbon excels in demanding applications where multiple, often conflicting, material properties are required simultaneously.
Summary Table:
| Characteristic | Description |
|---|---|
| Thermal Stability | Stable up to 3400°C in inert atmospheres. |
| Chemical Inertness | Highly resistant to chemical attack and oxidation. |
| Hardness | Approaches diamond-like hardness, resistant to wear. |
| Electrical Conductivity | Good conductor with a wide electrochemical potential window. |
| Structure | Amorphous, isotropic, and gas-tight. |
| Key Limitation | Brittle material, requires careful handling and design. |
Need a material that combines chemical inertness, high-temperature stability, and electrical conductivity?
Glassy carbon is the ideal solution for demanding applications in electrochemistry, high-temperature processing, and sensor technology where other materials fail. KINTEK specializes in providing high-quality lab equipment and consumables, including glassy carbon components, to meet the precise needs of your laboratory.
Let us help you enhance your research and processes. Contact our experts today to discuss how our glassy carbon solutions can benefit your specific application.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- How should a glassy carbon electrode be stored during long periods of non-use? Ensure Peak Performance & Longevity
- What is the typical working electrode potential range for a glassy carbon electrode in aqueous electrolytes? A Guide to Accurate Electrochemical Measurements
- What is the proper post-treatment and storage procedure for a glassy carbon electrode? Ensure Reliable, Reproducible Results
- Why is glassy carbon selected for mediator-assisted indirect oxidation of glycerol? The Key to Unbiased Research
- How to make a glassy carbon electrode? A Guide to the Industrial Pyrolysis Process



















