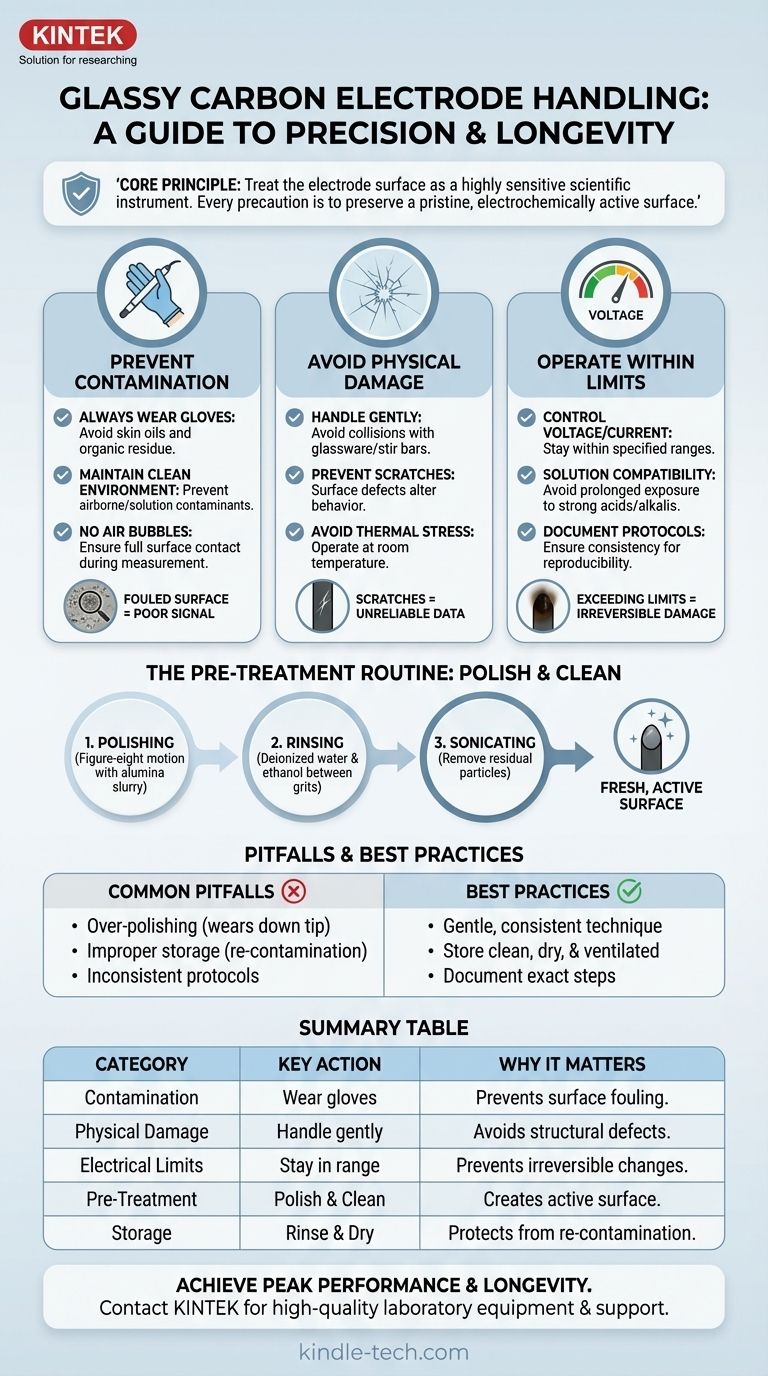
To handle a glassy carbon electrode correctly, you must prioritize three areas: preventing surface contamination, avoiding physical damage, and operating within its specified electrical limits. Because the electrode's performance is entirely dependent on its surface condition, you must handle it with meticulous care—avoiding skin contact, scratches, and exposure to incompatible chemicals or excessive voltage.
The core principle of handling a glassy carbon electrode is treating its surface as a highly sensitive scientific instrument. Every precaution, from polishing to storage, is designed to preserve a pristine, electrochemically active surface to ensure your measurements are accurate and reproducible.

The Foundation: Why Surface Integrity is Everything
A glassy carbon electrode (GCE) is valued for its wide potential window, chemical inertness, and high conductivity. However, these benefits are only realized when its surface is perfectly clean and structurally intact. A compromised surface is the single biggest cause of unreliable electrochemical data.
Contamination is the Primary Enemy
The GCE surface is easily fouled by organic molecules, metallic compounds, and even oils from your skin. This contamination blocks active sites, leading to poor signal, sluggish electron transfer, and inaccurate results.
Always wear gloves when handling the electrode to prevent transferring skin oils. Maintain a clean experimental environment to avoid airborne or solution-based contaminants.
The Material is Inherently Brittle
Glassy carbon is hard but also brittle. Careless handling can easily lead to microscopic scratches or even fractures.
Handle the electrode gently to avoid collisions with glassware or stir bars. Scratches create defects on the surface that can alter its electrochemical behavior and are difficult to remove completely.
Air Bubbles Invalidate Measurements
During an experiment, ensure no air bubbles adhere to the electrode face. A bubble effectively insulates that portion of the surface, reducing the active area and distorting the measured current.
Operating Within Safe Limits
Beyond physical handling, you must control the electrode's chemical and electrical environment to prevent irreversible damage.
Control Voltage and Current
Always operate the electrode within the specified current and voltage limits recommended for your system. Exceeding these limits can cause irreversible changes to the carbon structure or damage the electrode body.
Avoid Thermal Stress
High temperatures can alter the microstructure of glassy carbon. Operate at room temperature and never expose the electrode to an external heat source.
Ensure Solution Compatibility
While chemically inert, prolonged exposure to harsh solutions can degrade the electrode. Avoid immersing the GCE in strong acid or strong alkali solutions for extended periods.
The Non-Negotiable Pre-Treatment Routine
An electrode is never ready for a sensitive measurement straight out of the box or after previous use. A rigorous cleaning and polishing protocol is essential.
Why Pre-Treatment is Essential
The goal of pre-treatment is to remove any existing contamination and create a smooth, active, and reproducible surface for your experiment. This process typically involves polishing followed by cleaning.
The Polishing Method
Polishing mechanically removes a microscopic layer from the surface, exposing a fresh, active plane.
- Secure a polishing cloth (e.g., nylon or microfiber) to a flat plate.
- Apply a small amount of alumina powder slurry (typically starting with ~1.0 µm and finishing with ~0.05 µm).
- Hold the electrode perpendicular to the cloth and polish in a figure-eight motion.
- Rinse the electrode thoroughly with deionized water and ethanol between grit sizes and after the final polish.
Final Cleaning and Rinsing
After polishing, sonicate the electrode in deionized water or ethanol to remove any residual alumina particles. For some applications, an electrochemical activation step (cycling the potential in a supporting electrolyte) may be necessary.
Common Pitfalls and Best Practices
Avoiding common mistakes is as important as following the correct procedures. This is where experimental reproducibility is won or lost.
Over-Polishing vs. Under-Polishing
Aggressive or excessive polishing can wear down the electrode tip over time. Conversely, insufficient polishing will fail to remove deep contamination, leaving the surface inactive. The key is to find a consistent, gentle technique that renews the surface without removing excess material.
Improper Storage Undoes Your Work
After cleaning, your electrode is highly susceptible to re-contamination. Immediately rinse the surface with deionized water and then ethanol, and allow it to air dry.
Store the electrode in a clean, dry, and ventilated container, protected from lab fumes, dust, and moisture. Storing it improperly will contaminate the surface you just worked to prepare.
Inconsistent Protocols
The most significant source of experimental irreproducibility is an inconsistent pre-treatment protocol. Document your exact polishing and cleaning steps and apply them identically before every single experiment.
Making the Right Choice for Your Goal
Your specific application will determine the level of rigor required.
- If your primary focus is routine analysis: Establish a consistent, documented pre-treatment protocol and follow it every time to ensure your results are comparable from day to day.
- If your primary focus is high-sensitivity sensing: Meticulous polishing with fine alumina, thorough rinsing, and electrochemical performance verification with a standard (like potassium ferricyanide) are non-negotiable.
- If your primary focus is longevity and cost-saving: Prioritize careful physical handling, operation within safe electrical limits, and proper storage to prevent irreversible damage.
By treating the electrode's surface with disciplined care, you ensure the integrity and reproducibility of your electrochemical data.
Summary Table:
| Precaution Category | Key Action | Why It Matters |
|---|---|---|
| Prevent Contamination | Always wear gloves; avoid skin contact. | Skin oils and organic molecules foul the surface, blocking active sites and ruining data. |
| Avoid Physical Damage | Handle gently; prevent collisions and scratches. | Glassy carbon is brittle. Scratches create defects that alter electrochemical behavior. |
| Control Electrical Limits | Operate within specified voltage/current ranges. | Exceeding limits can cause irreversible damage to the carbon structure. |
| Ensure Proper Pre-Treatment | Polish with alumina slurry in a figure-eight motion. | Creates a fresh, active, and reproducible surface for accurate measurements. |
| Implement Correct Storage | Rinse with water/ethanol; store in a clean, dry container. | Protects the pristine surface from lab fumes and dust after cleaning. |
Achieve Peak Performance and Longevity for Your Glassy Carbon Electrodes
Maximizing the lifespan and accuracy of your sensitive lab equipment is critical. KINTEK specializes in providing high-quality laboratory equipment and consumables, including the precise tools needed for proper electrode maintenance.
Let us help you ensure your electrochemical data is always reliable. Contact our experts today to discuss your specific laboratory needs and discover how our solutions can support your research integrity.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What are the functions of a glassy carbon electrode in CV testing of antioxidants? Enhance Your Redox Analysis Accuracy
- What is a glassy carbon electrode made of? The Engineered Material Powering Electrochemical Analysis
- What is the proper post-treatment and storage procedure for a glassy carbon electrode? Ensure Reliable, Reproducible Results
- Why is glassy carbon selected for mediator-assisted indirect oxidation of glycerol? The Key to Unbiased Research
- Why is a glassy carbon disc electrode an indispensable consumable? Ensure Reliable Catalyst Evaluation Today



















