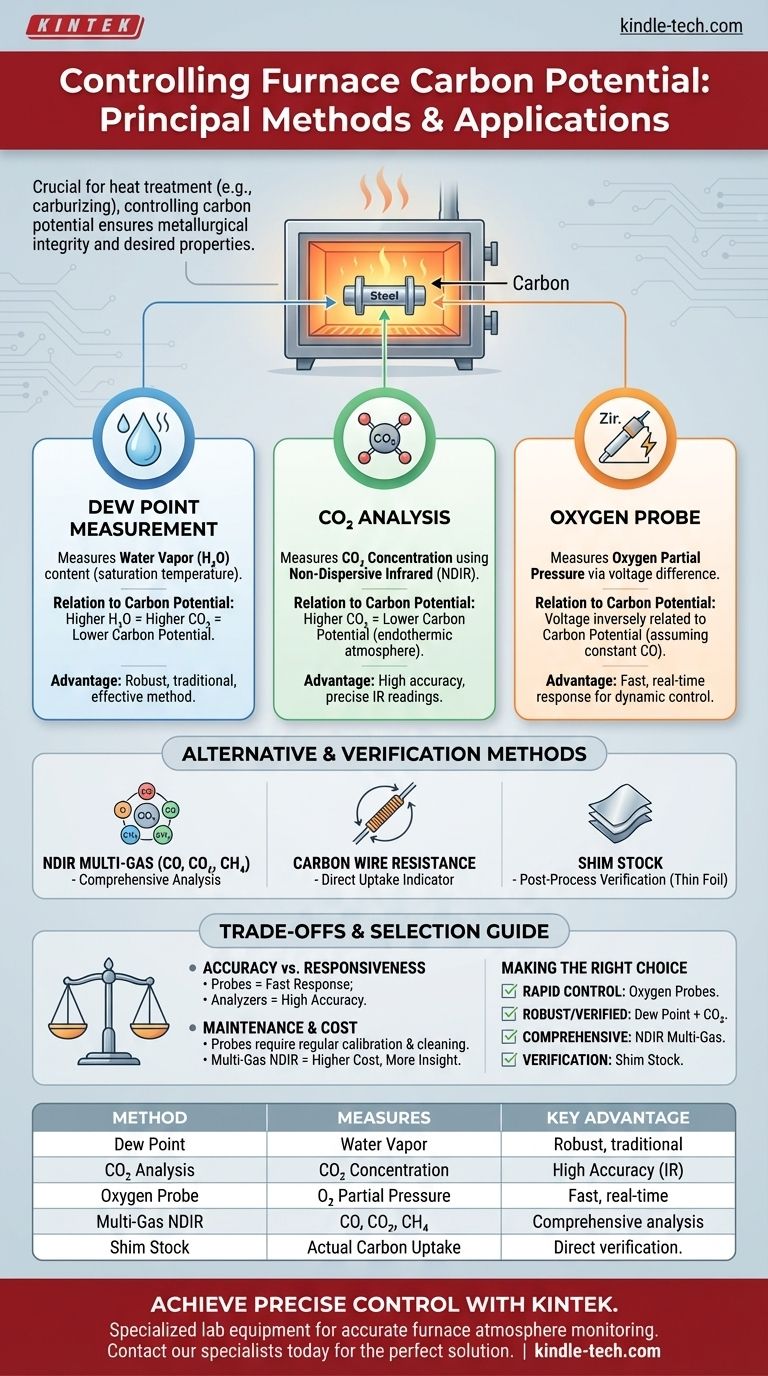
Controlling the carbon potential of a furnace atmosphere primarily relies on monitoring specific gas species that indicate the atmosphere's carburizing or decarburizing tendency. The most widely recognized and employed methods include measuring the dew point (water vapor content), analyzing carbon dioxide (CO2) levels using infrared analyzers, and determining oxygen partial pressure via oxygen or carbon probes. These techniques allow for real-time adjustments to maintain the desired carbon transfer to the metal.
Maintaining precise carbon potential is crucial for heat treatment processes like carburizing. It directly influences the surface hardness, wear resistance, and overall metallurgical properties of the treated steel, requiring careful selection and application of monitoring technologies to achieve specific material outcomes.

Understanding Carbon Potential
Carbon potential is a measure of an atmosphere's ability to transfer carbon to or from a steel surface at a given temperature. In heat treatment, particularly carburizing, it dictates the carbon content that the surface of the steel will absorb. This is a critical parameter for achieving desired material properties.
Why Control is Essential
Precise control of carbon potential prevents undesirable outcomes. Too high a carbon potential can lead to excessive carbon absorption and carbide formation, causing brittleness. Too low can result in insufficient carburization or even decarburization, failing to achieve the required hardness.
Factors Determining Target Carbon Potential
The ideal carbon potential for a process is not universal. It is specifically determined by:
- Steel Type: Different steel alloys have varying carbon absorption characteristics.
- Process Temperature: Temperature significantly influences the kinetics of carbon transfer and solubility.
Principal Methods for Carbon Potential Control
Several established methods are used to monitor and control the furnace atmosphere, each leveraging different gas properties.
Measuring Dew Point
Dew point measurement is a traditional and effective method.
How it Works
It directly measures the amount of water vapor (H2O) present in the furnace atmosphere. The dew point is the temperature at which air becomes saturated with water vapor, and condensation begins.
Relation to Carbon Potential
The water-gas shift reaction (CO + H2O ⇌ CO2 + H2) and the carburizing reaction (2CO ⇌ C + CO2) are central. Higher water vapor content (higher dew point) indicates a higher CO2 concentration, suggesting a lower carbon potential (more decarburizing).
Carbon Dioxide (CO2) Analysis
Infrared analyzers are commonly used for CO2 measurement.
How it Works
Non-Dispersive Infrared (NDIR) analyzers measure the absorption of infrared light by CO2 molecules in the gas sample. This provides a direct reading of the CO2 concentration.
Relation to Carbon Potential
In an endothermic atmosphere, the ratio of CO to CO2 is a strong indicator of carbon potential. An increase in CO2 generally means a decrease in carbon potential.
Oxygen Partial Pressure Measurement
Oxygen probes, also known as carbon probes, offer a direct electrical measurement.
How it Works
An oxygen probe typically consists of a yttrium-doped zirconia tube with platinum electrodes. One side is exposed to the furnace atmosphere, and the other to a reference air supply.
Measuring Voltage
The difference in oxygen partial pressure between the furnace atmosphere and the reference air generates a small voltage across the electrodes.
Relation to Carbon Potential
This voltage is directly correlated to the oxygen partial pressure in the furnace. This pressure is inversely related to the carbon potential, assuming a constant carbon monoxide (CO) content (typically around 20%).
Alternative and Verification Methods
While the primary methods provide real-time control, other techniques are used for monitoring, verification, or in specific applications.
NDIR Multi-Gas Analysis
Advanced NDIR systems can simultaneously measure multiple gas constituents.
Comprehensive Gas Composition
These analyzers can measure CO, CO2, and CH4 (methane), providing a more complete picture of the atmosphere's composition.
Enhanced Control
Knowing the concentrations of these key gases allows for more precise calculation and control of carbon potential, especially in dynamic processes.
Carbon Wire Resistance Analysis
This method involves a wire specifically chosen for its resistance change with carbon absorption.
Direct Carbon Uptake Indication
A small wire, often made of a material like iron, is exposed to the furnace atmosphere. As it absorbs carbon, its electrical resistance changes.
Real-time Monitoring
This change in resistance can be continuously monitored, providing a direct indication of the atmosphere's carburizing power.
Shim Stock Analysis
Shim stock analysis is a physical, post-process verification method.
Measuring Carbon Uptake
Thin steel foils (shims) of known carbon content are exposed to the furnace atmosphere. After a set time, they are removed.
Post-Process Verification
The shims are then analyzed (e.g., by weight change or combustion analysis) to determine the actual carbon absorbed. This validates the effectiveness of the atmosphere control.
Understanding the Trade-offs
Each method for controlling carbon potential has advantages and limitations that influence its suitability for different applications.
Accuracy vs. Responsiveness
Oxygen probes offer very fast response times, ideal for dynamic control, but can be sensitive to contamination. Dew point and CO2 analyzers are highly accurate but may have slightly slower response.
Maintenance and Calibration
Probes and sensors require regular calibration and can be susceptible to fouling by soot or other furnace contaminants, necessitating periodic cleaning or replacement.
Cost and Complexity
The initial investment and ongoing operational costs vary. Multi-gas NDIR systems, while comprehensive, are generally more expensive than basic dew point or CO2 analyzers.
Making the Right Choice for Your Goal
Selecting the optimal control method depends on the specific requirements of your heat treatment process and the desired level of precision.
- If your primary focus is rapid, dynamic control: Oxygen probes are highly effective due to their fast response time to atmosphere changes.
- If your primary focus is robust, well-established control with cross-verification: Combining dew point measurement with CO2 infrared analysis provides a reliable and redundant system.
- If your primary focus is comprehensive atmosphere understanding for complex processes: An NDIR multi-gas analyzer offers the most detailed insight into CO, CO2, and CH4 levels for advanced control strategies.
- If your primary focus is verification of actual carbon transfer to material: Periodic shim stock analysis is invaluable for confirming the efficacy of your control system.
The accurate control of carbon potential ensures metallurgical integrity and performance, making the judicious selection and maintenance of these atmospheric control methods paramount.
Summary Table:
| Method | Measures | Key Advantage |
|---|---|---|
| Dew Point | Water Vapor (H₂O) | Robust, traditional method |
| CO₂ Analysis | Carbon Dioxide (CO₂) | High accuracy with IR analyzers |
| Oxygen Probe | Oxygen Partial Pressure | Fast, real-time response |
| Multi-Gas NDIR | CO, CO₂, CH₄ | Comprehensive atmosphere analysis |
| Shim Stock | Actual Carbon Uptake | Direct post-process verification |
Achieve precise carbon potential control for superior metallurgical results.
KINTEK specializes in supplying the advanced lab equipment and consumables necessary for accurate furnace atmosphere monitoring. Whether you need robust oxygen probes, high-accuracy infrared analyzers, or verification tools like shim stock, our solutions are designed to meet the stringent demands of laboratory heat treatment processes.
Let our expertise help you enhance your material properties and process reliability.
Contact our specialists today to discuss your specific application and find the perfect control solution for your lab.
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the application of reducing atmospheres in metalworking? Achieve Perfect Surface Integrity in Heat Treatment
- What is the purpose of maintaining a continuous argon flow? Optimize PLAP Recovery with High Purity Aluminum
- What is the role of a program-controlled carbonization furnace in the preparation of lignin-based carbon fiber? Explained
- What is the function of a high-temperature atmosphere furnace in the heat treatment of 300M steel? Achieve Precision
- Why is an atmosphere tube furnace necessary for carbon-coated silicon anodes? Ensure Peak Material Purity
- What is the primary function of a high-rate atmosphere annealing furnace? Achieve Stoichiometric MOx Fuel Precision
- What is a controlled atmosphere furnace for heat treatment? Master Surface Chemistry and Metallurgy
- What are the 6 inert gases in air? A Guide to Noble Gases and Their Practical Uses



















