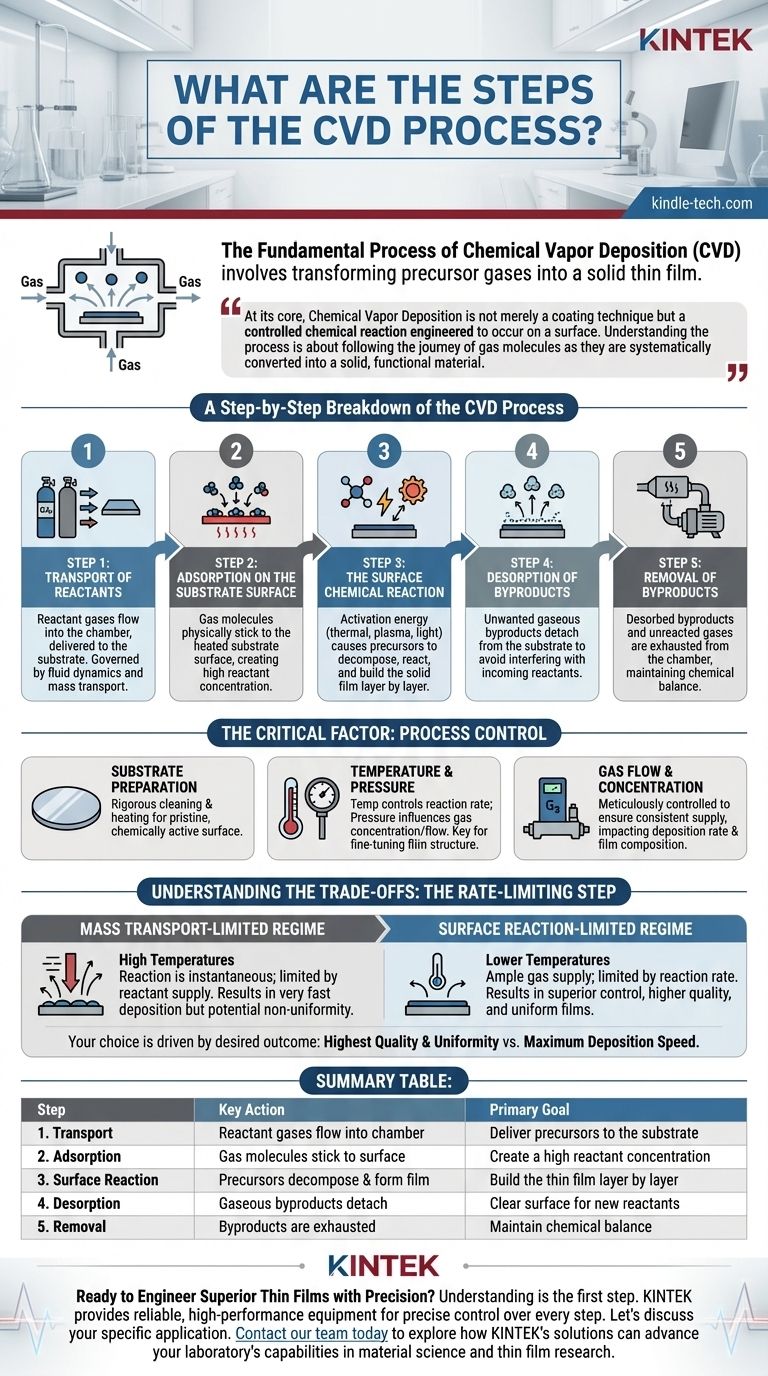
The fundamental process of Chemical Vapor Deposition (CVD) involves a series of sequential events that transform precursor gases into a solid thin film on a substrate. It begins with the transport of reactant gases into a reaction chamber, followed by their adsorption onto the substrate's surface. A chemical reaction is then induced on the surface, typically by heat, forming the desired film and gaseous byproducts, which are then removed from the chamber.
At its core, Chemical Vapor Deposition is not merely a coating technique but a controlled chemical reaction engineered to occur on a surface. Understanding the process is about following the journey of gas molecules as they are systematically converted into a solid, functional material.

A Step-by-Step Breakdown of the CVD Process
The CVD process can be universally understood as a five-step sequence. While different systems and materials introduce specific variables, these core mechanistic steps remain constant.
Step 1: Transport of Reactants
The process begins by introducing a controlled mixture of reactant gases (precursors) and inert diluent or carrier gases into the reaction chamber. These gases flow towards the substrate, the material to be coated. This phase is governed by principles of fluid dynamics and mass transport, as the concentration of reactants near the substrate is critical.
Step 2: Adsorption on the Substrate Surface
As the reactant gas molecules reach the substrate, they physically stick to its surface in a process called adsorption. This is a temporary attachment, creating a high concentration of reactant molecules directly on the surface where the film will be formed. The substrate is typically heated to facilitate the upcoming reaction.
Step 3: The Surface Chemical Reaction
This is the heart of the CVD process. With the aid of an activation energy source—most commonly thermal energy from the heated substrate, but also potentially plasma or light—the adsorbed precursor molecules decompose and react. This chemical transformation builds the solid film layer by layer, a process involving nucleation (the initial formation of stable clusters) and growth.
Step 4: Desorption of Byproducts
The chemical reactions that form the solid film almost always produce unwanted gaseous byproducts. These byproducts must detach, or desorb, from the substrate surface. If they fail to leave promptly, they can interfere with the incoming reactants and compromise the quality of the growing film.
Step 5: Removal of Byproducts
Finally, the desorbed gaseous byproducts, along with any unreacted precursor gases, are transported away from the substrate. They are then exhausted from the reaction chamber, typically by a vacuum system. This continuous removal is essential to maintain the chemical balance required for stable deposition.
The Critical Factor: Process Control
The quality, thickness, and properties of the final film are not accidental; they are dictated by precise control over the chamber environment. Simply following the steps is not enough.
Substrate Preparation
The process is highly sensitive to the condition of the substrate. Before deposition begins, substrates undergo rigorous cleaning and heating cycles inside the chamber to remove any moisture or contaminants. A pristine, chemically active surface is essential for uniform film growth.
Temperature and Pressure
Temperature is the primary lever for controlling the rate of the surface reaction. Pressure, in turn, influences the concentration and flow of reactant gases. The interplay between these two parameters is the main tool used to fine-tune the film's structure and properties.
Gas Flow and Concentration
The flow rates and partial pressures of each precursor gas must be meticulously controlled. This ensures a consistent supply of reactants to the substrate surface, directly impacting the deposition rate and the chemical composition of the final film.
Understanding the Trade-offs: The Rate-Limiting Step
In any multi-step process, one step is always the slowest, acting as a bottleneck that determines the overall speed. In CVD, this "rate-limiting step" dictates the entire deposition outcome.
Mass Transport-Limited Regime
At very high temperatures, the surface reaction happens almost instantaneously. The process speed is therefore limited by how fast you can supply fresh reactant gases to the surface. This mass transport-limited operation allows for very fast deposition but can often result in non-uniform films.
Surface Reaction-Limited Regime
At lower temperatures, there is an ample supply of reactant gas at the surface, but the chemical reaction itself is the bottleneck. This surface reaction-limited regime is slower but offers far superior control. It allows the molecules to find the ideal sites to bond, resulting in higher-quality, more uniform, and less defective films.
How to Apply This to Your Goal
Your choice of process parameters should be driven by the desired outcome for your film.
- If your primary focus is the highest quality and uniformity: You must operate in the surface reaction-limited regime, which typically involves lower temperatures and precise control over gas concentrations.
- If your primary focus is maximum deposition speed: You will need to operate in the mass transport-limited regime, using higher temperatures and gas flow rates, while accepting the potential trade-off in film uniformity.
By mastering the control of these fundamental steps, you can precisely engineer materials atom by atom.
Summary Table:
| Step | Key Action | Primary Goal |
|---|---|---|
| 1. Transport | Reactant gases flow into the chamber | Deliver precursors to the substrate |
| 2. Adsorption | Gas molecules stick to the substrate surface | Create a high reactant concentration |
| 3. Surface Reaction | Precursors decompose & form the solid film | Build the thin film layer by layer |
| 4. Desorption | Gaseous byproducts detach from the surface | Clear the surface for new reactants |
| 5. Removal | Byproducts are exhausted from the chamber | Maintain chemical balance for stable deposition |
Ready to Engineer Superior Thin Films with Precision?
Understanding the CVD process is the first step. Implementing it with reliable, high-performance equipment is what delivers results. At KINTEK, we specialize in providing the lab equipment and consumables that give you precise control over every step—from gas flow and temperature to pressure—ensuring your deposition process is optimized for quality, uniformity, and speed.
Let's discuss your specific application. Whether you are focused on achieving the highest film quality or maximizing deposition rates, our experts can help you select the right system and parameters for your goals.
Contact our team today to explore how KINTEK's solutions can advance your laboratory's capabilities in material science and thin film research.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What materials are used in CVD coating? Discover Hard Nitrides, Silicon Compounds & Diamond Films
- What is CVD coating? Transform Your Material's Surface for Maximum Performance
- Why is a UHVCVD environment necessary for ruthenium-based thin film deposition? Ensuring High Purity & Conductivity
- What is the advantage of magnetically assisted sputtering? Achieve Faster, Purer Thin-Film Deposition
- What is CVD in nanomaterials? A Guide to High-Purity Material Fabrication
- What are the steps of sputtering? A Guide to Thin Film Deposition
- Why do we do sputtering? Achieve Superior Thin Film Quality and Adhesion
- What is the vapor transport deposition process? PVD vs. CVD Explained



















