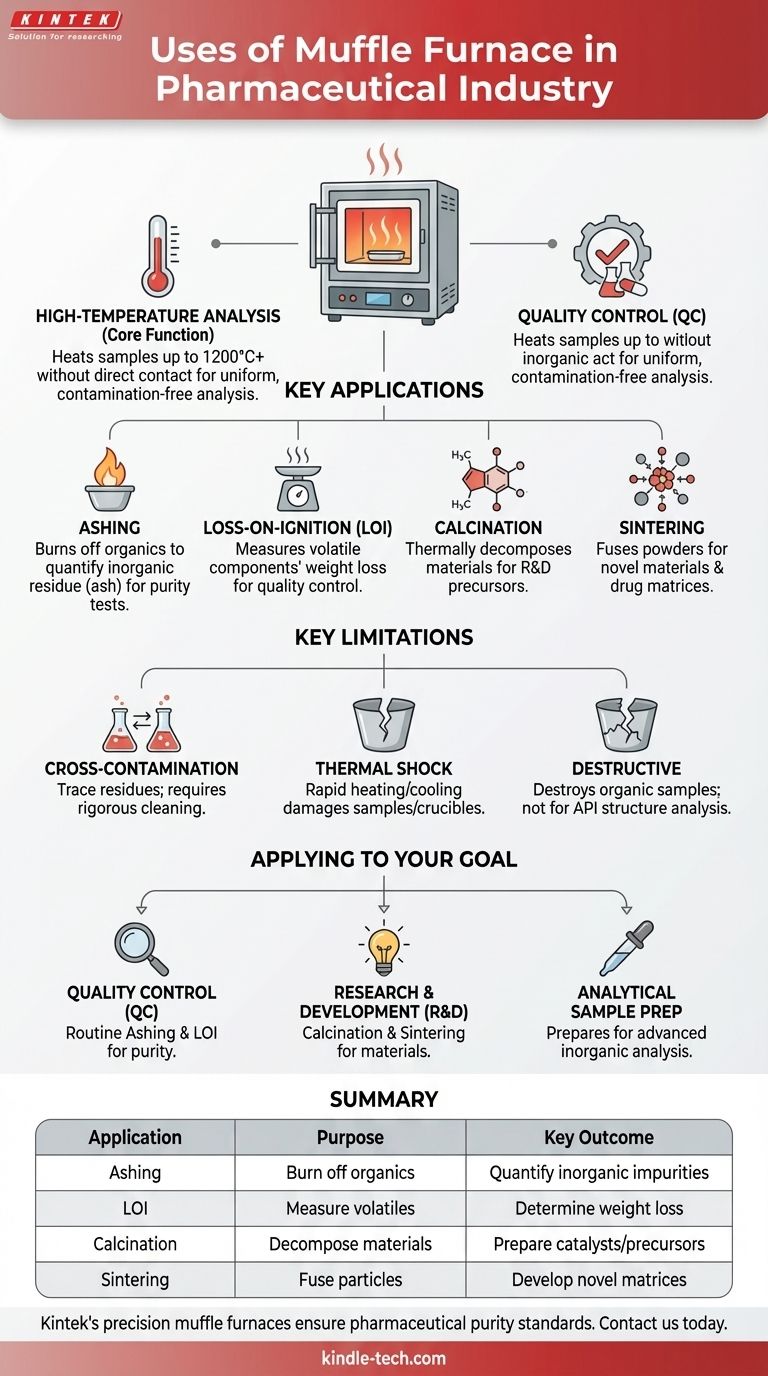
In the pharmaceutical industry, a muffle furnace is primarily used for the high-temperature preparation and analysis of samples, which is a critical step in drug inspection and quality control. These furnaces provide the precise, controlled heat needed to burn away organic compounds, allowing technicians to isolate and quantify the inorganic residue left behind.
A muffle furnace's role extends beyond simple heating; it is an essential analytical tool for ensuring drug purity and safety. Its primary function is to perform ashing and loss-on-ignition tests, which are fundamental for quantifying inorganic impurities and meeting stringent pharmacopoeial standards.

The Core Function: High-Temperature Analysis
A muffle furnace is essentially a high-temperature oven, insulated to reach temperatures up to 1200°C or higher. Its key feature is that the sample is heated without coming into direct contact with the heating element, preventing contamination and ensuring uniform temperature.
Ashing for Impurity Analysis
Ashing is the most common pharmaceutical application. The process involves heating a sample at a very high temperature to completely burn off all organic substances, including the active pharmaceutical ingredient (API) and excipients.
What remains is the inorganic residue or "ash." Weighing this residue is a direct measure of the total amount of inorganic impurities in the original sample. This procedure is the basis for standard pharmacopoeial tests like "Residue on Ignition" or "Sulphated Ash."
Loss-on-Ignition (LOI) Testing
Loss-on-ignition is a specific analytical method that uses a muffle furnace to determine the weight percentage of volatile components in a sample.
The sample is weighed before and after the heating process. The weight lost during ignition corresponds to components like water, carbonates, or other volatile materials, providing critical data for quality control.
Calcination for Material Preparation
Calcination involves heating a solid material to a high temperature to cause thermal decomposition or drive off volatile substances, but without melting it.
In pharmaceutical R&D, this can be used to synthesize stable inorganic materials or catalysts. For example, it can convert metal carbonates into their more reactive oxide forms for use in subsequent chemical processes.
Sintering in Materials Science
Sintering uses heat to fuse particles of a powder together, creating a solid or porous solid mass. This is done below the material's melting point.
While less common, this application is relevant in pharmaceutical materials science for creating specific ceramic components or developing novel drug delivery matrices.
Understanding the Key Limitations
While essential, operating a muffle furnace requires a clear understanding of its limitations to ensure accurate and safe results.
Risk of Cross-Contamination
The interior chamber can retain trace residues from previous runs. Without rigorous cleaning protocols between different sample types, cross-contamination can occur, leading to inaccurate impurity measurements.
Thermal Shock to Samples and Crucibles
Rapid heating or cooling can cause thermal shock, which can damage or even shatter the ceramic crucibles used to hold samples. It may also cause certain samples to spatter, resulting in material loss and inaccurate final weights. Controlled, gradual temperature ramps are essential.
Destructive to Organic Components
By its very nature, the ashing process destroys the organic sample. It is a tool for analyzing what is not the drug. It cannot be used to analyze the chemical structure or properties of the active pharmaceutical ingredient itself.
Applying This to Your Pharmaceutical Goal
The specific use of a muffle furnace depends entirely on your team's objective within the drug lifecycle.
- If your primary focus is Quality Control (QC): Your main use will be performing routine ashing and loss-on-ignition tests to quantify inorganic impurities and ensure batches comply with pharmacopoeial standards.
- If your primary focus is Research & Development (R&D): You will use it for materials science applications, such as calcination to create precursor materials or sintering to develop novel formulations.
- If your primary focus is Analytical Sample Prep: The furnace is essential for preparing samples for more advanced inorganic analysis (e.g., Atomic Absorption Spectroscopy) by cleanly removing the organic matrix.
Ultimately, the muffle furnace is a foundational instrument for verifying the purity and quality that underpins pharmaceutical safety.
Summary Table:
| Application | Purpose | Key Outcome |
|---|---|---|
| Ashing | Burn off organic material | Quantify inorganic impurities (ash) |
| Loss-on-Ignition (LOI) | Measure volatile components | Determine weight loss for quality control |
| Calcination | Thermally decompose materials | Prepare inorganic catalysts or precursors |
| Sintering | Fuse powder particles | Develop novel drug delivery matrices |
Ensure your pharmaceutical products meet the highest purity standards with KINTEK's precision muffle furnaces. Our lab equipment is designed for accurate ashing, loss-on-ignition, and calcination processes, providing the reliable performance your quality control and R&D teams demand. Contact us today to find the perfect furnace for your laboratory's needs and enhance your drug safety protocols.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the use of burnout furnace? Create Flawless Castings with Precision Mold Preparation
- What are batch furnaces best used for? Maximize Flexibility in Heat Treatment & R&D
- What is the critical function of a high-temperature furnace in MEC carbon brush preparation? Optimize Bio-Anode Surface
- What is the temperature of a burnout furnace? A Guide to the Multi-Stage Cycle for Perfect Castings
- Why is a high-temp muffle furnace essential for ZnO-WO3 & ZnO-BiOI? Optimize Heterojunction Catalyst Performance
- What is the ash content of a furnace? The Tool vs. The Measurement Explained
- What is the primary role of a constant temperature oven in preparing thermochemical energy storage materials?
- What are the effects of different sintering temperatures? Mastering Density, Strength, and Component Integrity



















