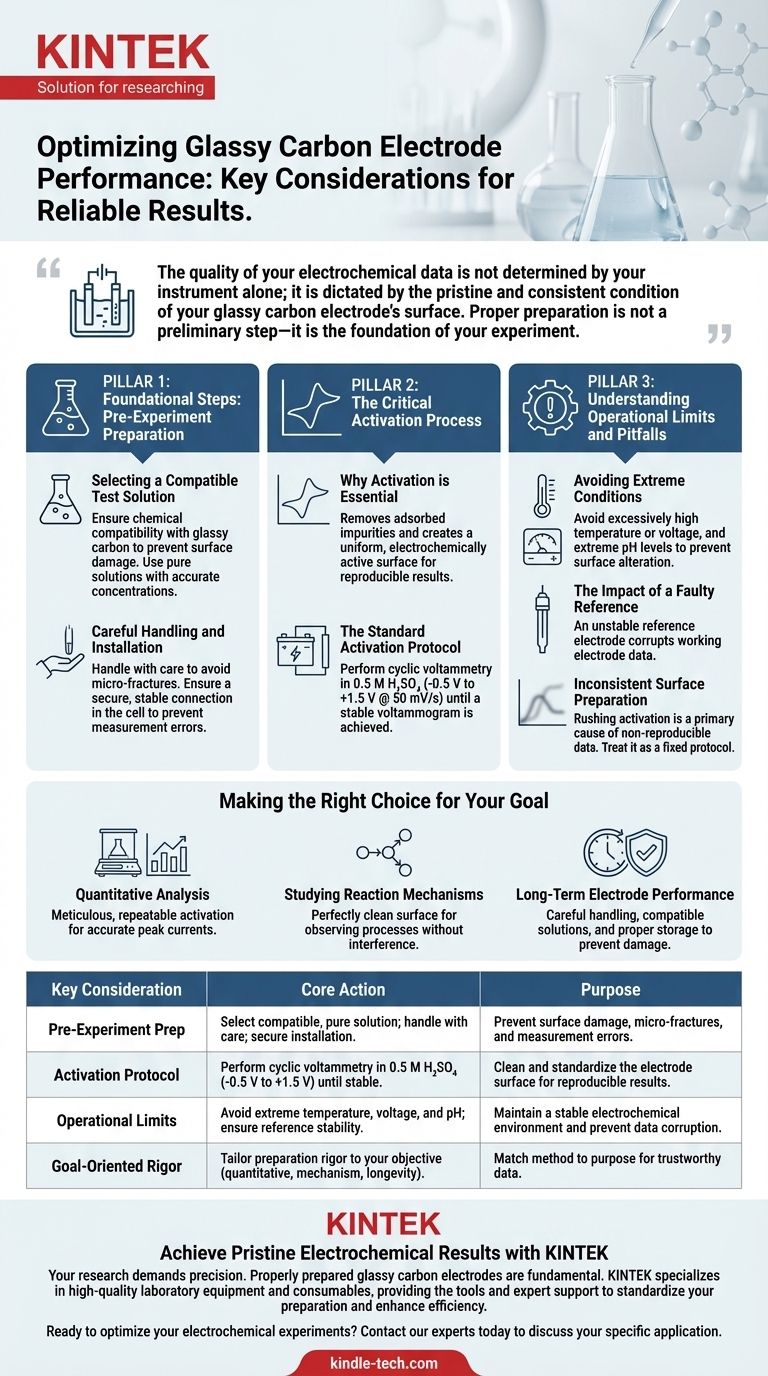
To ensure reliable results from a glassy carbon electrode, you must focus on three core areas: meticulous pre-experiment preparation, a consistent activation protocol, and careful handling during use. This involves selecting a compatible and pure test solution, installing the electrode securely to avoid measurement errors, and activating its surface via cyclic voltammetry until a stable response is achieved.
The quality of your electrochemical data is not determined by your instrument alone; it is dictated by the pristine and consistent condition of your glassy carbon electrode's surface. Proper preparation is not a preliminary step—it is the foundation of your experiment.

Foundational Steps: Pre-Experiment Preparation
The work you do before running your first scan has the greatest impact on the quality of your results. Overlooking these foundational steps is a common source of experimental error.
Selecting a Compatible Test Solution
The chemical environment must not harm the electrode. Always select a test solution that is chemically compatible with glassy carbon to prevent surface damage or degradation.
Furthermore, the purity and concentration of your solution must meet the specific requirements of your experiment to ensure accurate measurements.
Careful Handling and Installation
Glassy carbon is a brittle material. Handle the electrode with care to prevent any physical impact, drops, or torsion that could cause micro-fractures and compromise its integrity.
When installing the electrode into the electrochemical cell, ensure a secure and stable connection. A loose connection is a frequent cause of noise and other measurement errors.
The Critical Activation Process
An unactivated electrode surface is not a reliable analytical tool. Activation is a non-negotiable step for cleaning and standardizing the electrode surface to ensure reproducible results.
Why Activation is Essential
The process removes adsorbed impurities and creates a uniform, electrochemically active surface. Without this step, your measurements can be inconsistent and non-reproducible from one experiment to the next.
The Standard Activation Protocol
A common and effective method is to perform cyclic voltammetry in a 0.5 M H₂SO₄ solution.
The potential should be scanned between -0.5 V and +1.5 V at a scan rate of approximately 50 mV/s. Continue this process until the resulting voltammogram becomes stable and repeatable.
Understanding Operational Limits and Pitfalls
A successful experiment requires maintaining a stable environment for the entire electrochemical cell. Pushing beyond established limits can introduce variables that corrupt your data.
Avoiding Extreme Conditions
While every system has unique parameters, it is a best practice to avoid operating in conditions of excessively high temperature or voltage.
Similarly, extreme pH levels—whether highly acidic or highly alkaline—can alter the electrode surface or the behavior of your analyte, leading to unreliable results.
The Impact of a Faulty Reference
Your glassy carbon working electrode is measured against a reference electrode. If the reference electrode is unstable due to extreme conditions, the data collected from your working electrode will be fundamentally flawed.
Inconsistent Surface Preparation
The most common pitfall is inconsistent or incomplete activation. Rushing this step or changing the protocol between experiments is a primary cause of non-reproducible data. Treat the activation protocol as a fixed part of your experimental method.
Making the Right Choice for Your Goal
Your experimental objective should guide your level of rigor. Use these principles to match your preparation to your purpose.
- If your primary focus is quantitative analysis: Meticulous and repeatable activation is paramount to ensure your measured peak currents are accurate and reproducible.
- If your primary focus is studying reaction mechanisms: A perfectly clean and well-defined electrode surface is essential to guarantee you are observing the desired electrochemical process without interference from contaminants.
- If your primary focus is long-term electrode performance: Always handle the electrode with care, use only compatible solutions, and clean and store it properly after each use to prevent irreversible damage.
Ultimately, treating your glassy carbon electrode with methodical care is the surest path to producing trustworthy and defensible scientific data.
Summary Table:
| Key Consideration | Core Action | Purpose |
|---|---|---|
| Pre-Experiment Prep | Select a compatible, pure test solution; handle with care; ensure secure installation. | Prevent surface damage, micro-fractures, and measurement errors. |
| Activation Protocol | Perform cyclic voltammetry in 0.5 M H₂SO₄ (-0.5 V to +1.5 V) until stable. | Clean and standardize the electrode surface for reproducible results. |
| Operational Limits | Avoid extreme temperature, voltage, and pH levels; ensure reference electrode stability. | Maintain a stable electrochemical environment and prevent data corruption. |
| Goal-Oriented Rigor | Tailor preparation rigor to your objective (quantitative analysis, mechanism study, longevity). | Match method to purpose for trustworthy data. |
Achieve Pristine Electrochemical Results with KINTEK
Your research demands precision. Properly prepared glassy carbon electrodes are fundamental to obtaining accurate, reproducible electrochemical data—whether for quantitative analysis, reaction mechanism studies, or ensuring long-term electrode performance.
KINTEK specializes in supplying high-quality laboratory equipment and consumables. We provide the reliable tools and expert support you need to standardize your electrode preparation and enhance your lab's efficiency.
Ready to optimize your electrochemical experiments? Contact our experts today to discuss your specific application and discover how KINTEK can support your research goals.
Visual Guide
Related Products
- Glassy Carbon Electrochemical Electrode
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Gold Disc Electrode
People Also Ask
- How to make a glassy carbon electrode? A Guide to the Industrial Pyrolysis Process
- What are the functions of a glassy carbon electrode in CV testing of antioxidants? Enhance Your Redox Analysis Accuracy
- Why is a glassy carbon disc electrode an indispensable consumable? Ensure Reliable Catalyst Evaluation Today
- How should a glassy carbon electrode be stored during long periods of non-use? Ensure Peak Performance & Longevity
- What is the proper post-treatment and storage procedure for a glassy carbon electrode? Ensure Reliable, Reproducible Results



















