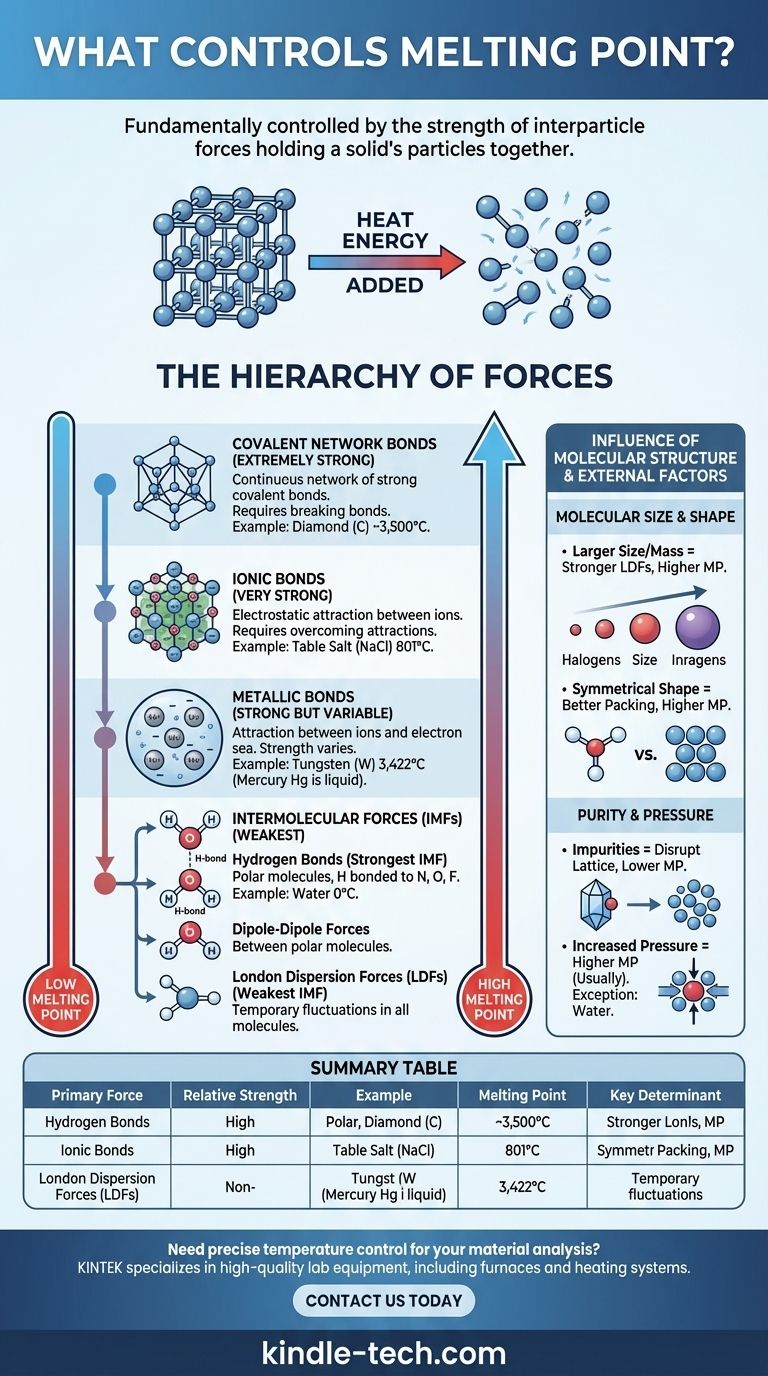
The melting point of a substance is fundamentally controlled by the strength of the forces holding its particles together. In a solid, particles (atoms, ions, or molecules) are locked in a fixed, ordered arrangement called a crystal lattice. To melt the solid, you must add enough heat energy to allow these particles to overcome those forces and move past one another as a liquid. Therefore, stronger forces require more energy to break, resulting in a higher melting point.
The core principle is simple: melting is not about breaking the particles themselves, but about overcoming the attractive forces between them. The type and strength of these interparticle forces—ranging from powerful ionic bonds to weak intermolecular attractions—are the primary determinants of a substance's melting point.

The Hierarchy of Forces
The immense variation in melting points—from the -259°C of hydrogen to the 3,422°C of tungsten—can be understood by classifying the forces holding a solid together. These forces exist in a clear hierarchy of strength.
Ionic Bonds (Very Strong)
In ionic compounds like table salt (NaCl), particles are positively and negatively charged ions. They are held together by powerful electrostatic attractions in a rigid crystal lattice.
Overcoming these strong ionic bonds requires a tremendous amount of thermal energy. Consequently, ionic compounds consistently exhibit very high melting points.
Covalent Network Bonds (Extremely Strong)
In covalent network solids, atoms are joined by a vast, continuous network of strong covalent bonds. There are no individual molecules; the entire crystal is essentially one giant molecule.
Substances like diamond (carbon) and quartz (silicon dioxide) are prime examples. To melt them, you must break these extremely strong covalent bonds, which demands enormous amounts of energy, leading to exceptionally high melting points.
Metallic Bonds (Strong but Variable)
Metals consist of a lattice of positive metal ions sitting in a "sea" of delocalized electrons that move freely throughout the structure. The attraction between the positive ions and this electron sea constitutes the metallic bond.
The strength of this bond, and thus the melting point, varies widely. It depends on factors like the charge of the ion and the number of delocalized electrons. This is why mercury is a liquid at room temperature, while tungsten has one of the highest melting points of any element.
Intermolecular Forces (Weakest)
For molecular compounds (like water, sugar, or wax), the forces that must be overcome for melting are intermolecular forces (IMFs)—the attractions between separate molecules. These are significantly weaker than the ionic, covalent, or metallic bonds discussed above.
There are three main types of IMFs:
- Hydrogen Bonds: The strongest type of IMF. It occurs in polar molecules where hydrogen is bonded directly to a highly electronegative atom (nitrogen, oxygen, or fluorine). Water's relatively high melting point (0°C) is due to these strong hydrogen bonds.
- Dipole-Dipole Forces: Occur between polar molecules that have permanent positive and negative ends. These forces are weaker than hydrogen bonds.
- London Dispersion Forces (LDFs): The weakest IMF, present in all molecules. They arise from temporary, random fluctuations in electron distribution. Though weak individually, their cumulative effect can be significant in larger molecules.
The Influence of Molecular Structure
Beyond the type of force, the specific size and shape of particles play a critical role, especially for molecular compounds.
Molecular Size and Mass
For compounds with the same primary intermolecular force (e.g., LDFs), larger molecules have higher melting points. This is because larger molecules have more electrons, creating a more "polarizable" electron cloud that leads to stronger London Dispersion Forces.
This trend is clear in the halogens: the melting point increases as you go from fluorine (F₂) to iodine (I₂).
Molecular Shape and Packing Efficiency
A molecule's ability to pack tightly and efficiently into a crystal lattice has a major impact. Symmetrical molecules often have higher melting points than less symmetrical isomers, even if they have the same formula and mass.
Symmetrical shapes allow molecules to fit together more closely in the solid state, maximizing the effectiveness of their intermolecular forces. Breaking this well-organized structure requires more energy.
Understanding the Trade-offs and Nuances
Predicting melting points involves weighing these interconnected factors.
Purity Changes Everything
The principles above assume a pure substance. Impurities disrupt the orderly crystal lattice, weakening the overall structure.
This disruption makes the solid easier to melt. As a result, an impure substance will melt at a lower temperature and over a broader range than its pure counterpart. This phenomenon is known as melting point depression.
Pressure Plays a Role
Melting points are typically stated at standard atmospheric pressure. For most substances, increasing pressure raises the melting point because it physically pushes the particles closer together, reinforcing the lattice structure.
Water is a famous and critical exception. Because solid ice is less dense than liquid water, increased pressure favors the denser liquid phase, thereby lowering the melting point.
How to Apply This to Your Analysis
When comparing substances, use a systematic approach to identify the key factors at play.
- If your primary focus is comparing different classes of solids: First, identify the primary binding force—ionic, covalent network, metallic, or intermolecular. This will give you the most significant indicator of relative melting point.
- If your primary focus is comparing two molecular compounds: Determine the strongest intermolecular force each one possesses (hydrogen bonds > dipole-dipole > LDFs). The compound with the stronger IMF will generally have a higher melting point.
- If your primary focus is on similar nonpolar molecules: The molecule with the greater mass and surface area will have stronger London Dispersion Forces and thus a higher melting point.
- If your primary focus is on isomers (same formula, different shape): The more symmetrical molecule that can pack more efficiently into a crystal lattice will often have a higher melting point.
By understanding this hierarchy of forces and the influence of structure, you can systematically explain the melting behavior of nearly any substance.
Summary Table:
| Primary Force Type | Relative Strength | Example Substance | Melting Point | Key Determinant |
|---|---|---|---|---|
| Covalent Network | Extremely Strong | Diamond (C) | ~3,500°C | Breaking covalent bonds in a continuous lattice |
| Ionic Bonds | Very Strong | Sodium Chloride (NaCl) | 801°C | Overcoming electrostatic attractions between ions |
| Metallic Bonds | Strong (Variable) | Tungsten (W) | 3,422°C | Strength of ion-electron sea attraction |
| Intermolecular Forces | Weakest | Water (H₂O) | 0°C | Hydrogen bonding, dipole-dipole, London dispersion forces |
Need precise temperature control for your material analysis? Understanding melting points is critical for reliable lab results. At KINTEK, we specialize in high-quality lab equipment, including furnaces and heating systems designed for accurate thermal analysis. Whether you're working with high-melting-point metals or sensitive molecular compounds, our solutions ensure consistent performance. Contact us today to discuss how our products can enhance your laboratory's capabilities and support your research. Reach out via our contact form to get started!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Which catalyst is used in pyrolysis process? Choosing the Right Catalyst for Your Feedstock
- How do laboratory high-temperature heating devices work with FT-IR to evaluate lubricant antioxidants? Expert Analysis
- What factors should be considered when selecting the size of an ultra-low freezer? A Guide to Maximizing Efficiency and Capacity
- What are the most common elemental analysis techniques? Choose the Right Tool for Your Material Analysis
- What is the function of a laboratory precision oven in GLYMO-rGO preparation? Ensure Optimal Nano-Filler Dispersion
- How does multizone heating work? Achieve Custom Comfort and Energy Savings
- What 5 safety precautions should be taken when heating anything in the lab? Essential Rules for Lab Safety
- Are there different types of annealing? Choose the Right Process for Your Metal



















