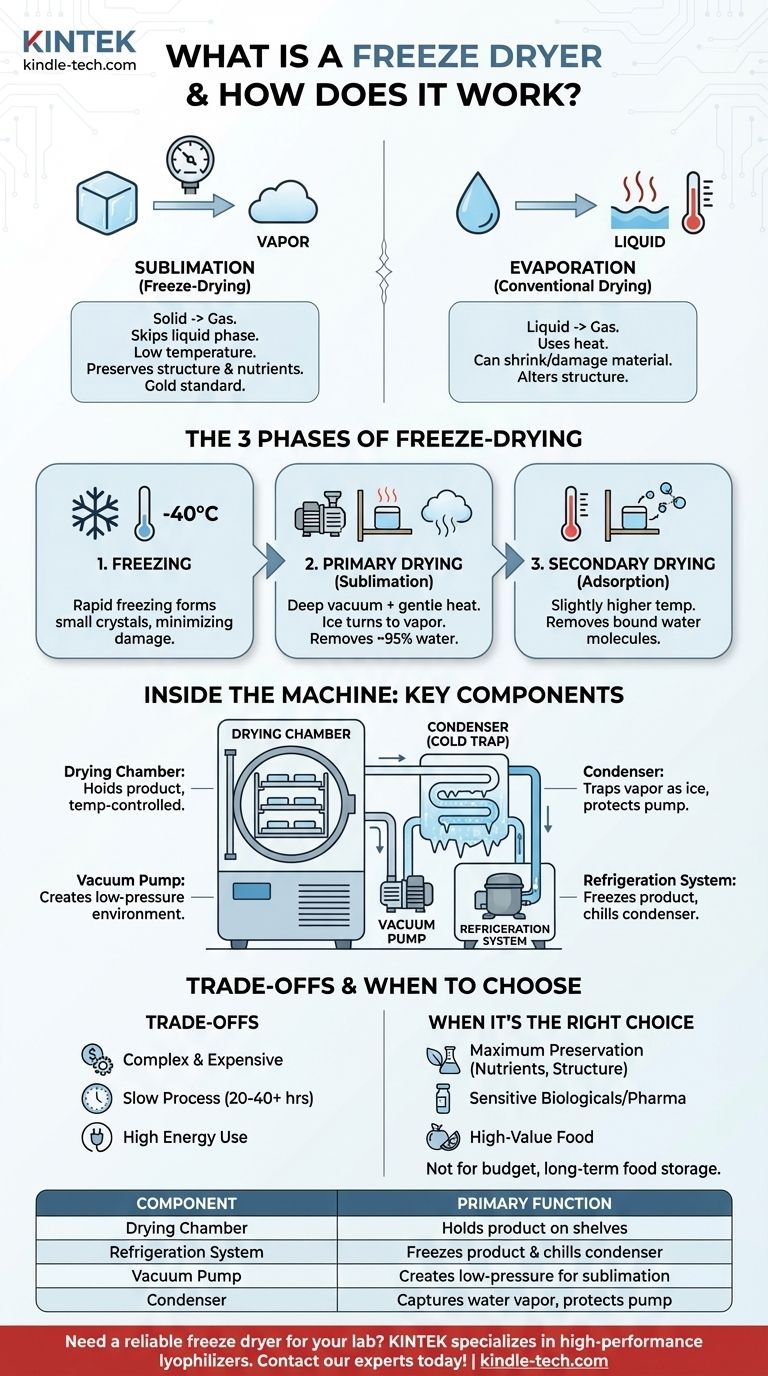
At its core, a freeze dryer is a specialized machine that removes water from a substance by first freezing it, then turning the ice directly into vapor under a vacuum. This process, called lyophilization or sublimation, is fundamentally different from simple dehydration and is renowned for its ability to preserve the original structure, nutritional value, and chemical integrity of the material.
The true purpose of a freeze dryer isn't merely to dry something—it's to preserve it. By bypassing the liquid water phase, it removes moisture without the destructive effects of heat, making it the gold standard for preserving sensitive materials from pharmaceuticals to high-quality food.

The Core Principle: Sublimation, Not Evaporation
What is Sublimation?
Sublimation is the physical process where a substance transitions directly from a solid state to a gas state, completely skipping the intermediate liquid phase.
In freeze-drying, the water within a product is first frozen into solid ice. Then, by creating a deep vacuum, the machine allows this ice to turn directly into water vapor, which is then collected and removed.
Why This Matters for Preservation
Conventional drying methods rely on evaporation, which uses heat to turn liquid water into vapor. This heat can shrink, toughen, and damage the delicate cellular structure of the material, often degrading nutrients, flavors, and aromas in the process.
Sublimation avoids this damage. Since the process occurs at low temperatures and the water is locked in place as ice, the material's original shape and structure remain intact. This creates a porous, lightweight final product that can be rehydrated almost instantly.
The Three Phases of Freeze-Drying
The entire process is a carefully controlled sequence designed to gently remove water without compromising the product.
Phase 1: Freezing
The material is first frozen, often rapidly and to a very low temperature (e.g., -40°C or colder).
The speed of this freezing phase is critical. Rapid freezing creates small ice crystals, which cause less damage to the cellular walls of the material compared to the large, sharp crystals formed during slow freezing.
Phase 2: Primary Drying (Sublimation)
Once frozen, the material is placed under a deep vacuum inside the freeze dryer's chamber. The pressure is reduced to a fraction of normal atmospheric pressure.
At this point, a small amount of heat is gently applied to the shelves holding the product. This provides just enough energy for the ice to sublimate into water vapor. This phase removes the vast majority of the water (around 95%).
Phase 3: Secondary Drying (Adsorption)
After the primary drying phase, a small amount of unfrozen water molecules may still be bound to the material's surface.
To remove this residual moisture, the temperature is raised slightly higher (still well below temperatures used in conventional drying) while the vacuum is maintained. This process, called adsorption, encourages the last of the water molecules to detach and be drawn away.
Inside the Machine: Key Components
A freeze dryer is a sophisticated system where several key parts work in concert.
The Drying Chamber
This is the sealed, insulated main body of the unit where the material is placed, usually on temperature-controlled shelves.
The Refrigeration System
This powerful system, which includes a compressor and evaporator, is responsible for two crucial tasks: freezing the product initially and, more importantly, chilling the condenser.
The Vacuum Pump
This is the heart of the sublimation process. The vacuum pump removes air from the drying chamber, creating the low-pressure environment necessary for ice to turn directly into vapor at low temperatures.
The Condenser (or Cold Trap)
The condenser is an extremely cold surface (often colder than the product itself) located within the vacuum system. As water vapor leaves the product, it is immediately attracted to the condenser, where it turns back into ice. This "traps" the water, preventing it from reaching and damaging the vacuum pump.
Understanding the Trade-offs
While highly effective, freeze-drying is not a one-size-fits-all solution.
The Cost of Precision
Freeze dryers are complex machines with refrigeration and high-vacuum systems, making them significantly more expensive to purchase and maintain than simple food dehydrators.
The Time Commitment
The freeze-drying process is methodical and slow. A typical cycle can take anywhere from 20 to 40+ hours to complete, depending on the material and its water content.
The Energy Consumption
Running a powerful refrigeration system and a vacuum pump for over 24 hours is an energy-intensive process. This contributes to a higher operational cost compared to other preservation methods.
When is Freeze-Drying the Right Choice?
Choosing this method depends entirely on your preservation goals.
- If your primary focus is maximum preservation of nutrients and structure: Freeze-drying is the unparalleled standard for sensitive biologicals, pharmaceuticals, high-value foods, or even precious documents.
- If your primary focus is simple, long-term food storage on a budget: Conventional dehydration is a more accessible and energy-efficient alternative, though it alters texture and can reduce nutrient content.
- If your goal is scientific research or pharmaceutical production: A laboratory or industrial freeze dryer (lyophilizer) is an essential, non-negotiable tool for stabilizing delicate samples like vaccines, antibodies, and cell cultures.
Ultimately, understanding the mechanics of freeze-drying empowers you to choose the precise preservation method that aligns with the value and sensitivity of your material.
Summary Table:
| Key Component | Primary Function |
|---|---|
| Drying Chamber | Holds the frozen product on temperature-controlled shelves under vacuum. |
| Refrigeration System | Freezes the product and chills the condenser to extremely low temperatures. |
| Vacuum Pump | Creates the low-pressure environment necessary for sublimation to occur. |
| Condenser (Cold Trap) | Captures and freezes water vapor, protecting the vacuum pump and removing moisture. |
Need a reliable freeze dryer for your lab? KINTEK specializes in high-performance laboratory equipment, including freeze dryers (lyophilizers) designed for precision and durability. Whether you're preserving sensitive pharmaceuticals, biological samples, or high-value food products, our solutions ensure maximum preservation of structure and integrity. Contact our experts today to find the perfect freeze-drying solution for your specific needs!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Liquid Nitrogen Cryogenic Grinder Mill Cryomill Airflow Ultrafine Pulverizer
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Small Injection Molding Machine for Lab Use
People Also Ask
- What is the primary purpose of an autoclave in the preparation of media for the biological leaching of uranium?
- Why is a 24-hour hydrothermal treatment in an autoclave necessary for BMO nanosheets? Unlock Superior Photocatalysis
- How does an autoclave ensure the reliability of experimental results? Achieving a Sterile Baseline for Lab Research
- Why is the prevention of air entrapment critical for the autoclave sterilization process? Ensure 100% Sterility Today
- What experimental conditions do stainless steel autoclaves provide for PCT-A leaching? Optimize Phosphate Glass Testing



















