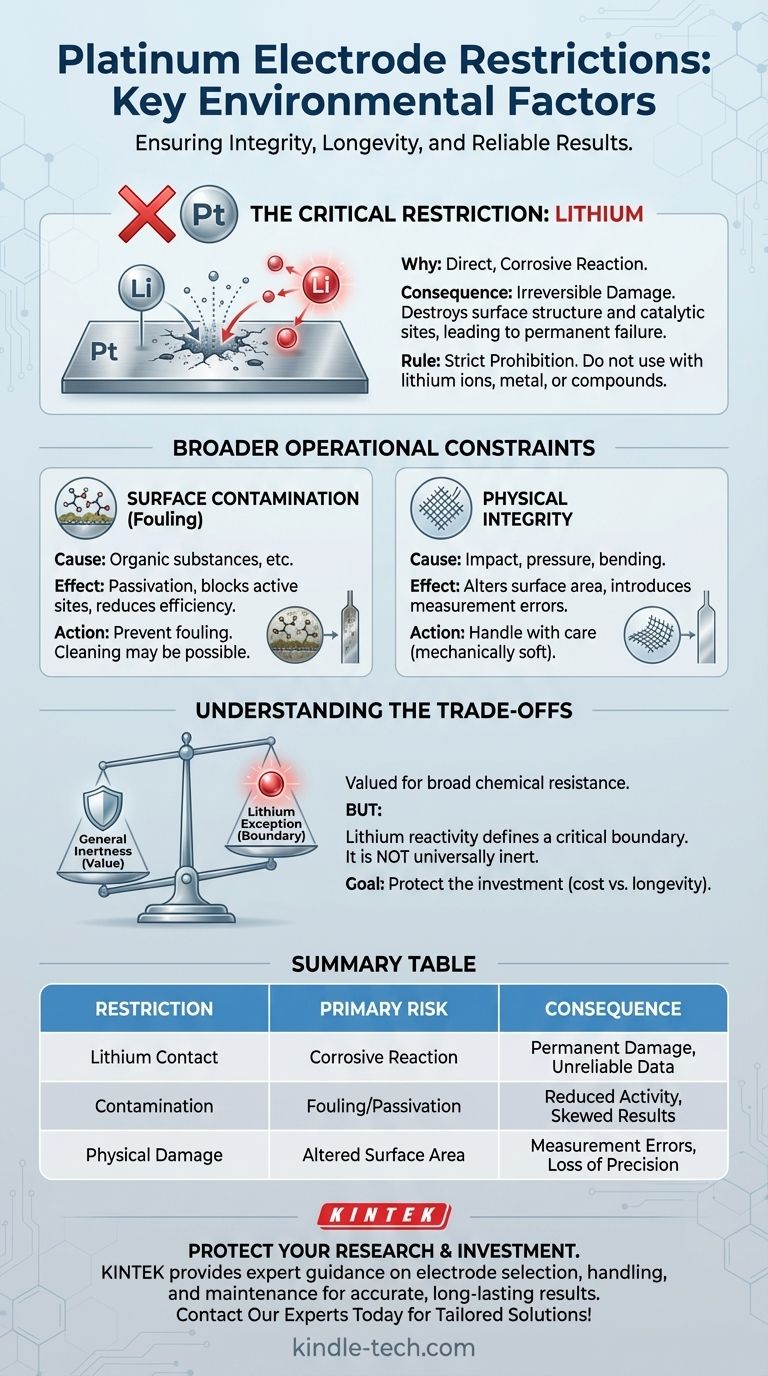
A critical environmental restriction for using platinum electrodes is the strict prohibition of any contact with lithium. While known for its general stability, platinum reacts directly with and is corroded by lithium, causing irreversible damage to the electrode and compromising experimental integrity.
While platinum is valued for its broad chemical inertness, its specific reactivity with lithium creates a significant operational boundary. Understanding this limitation, along with other potential sources of contamination and physical damage, is essential for maintaining the electrode's integrity and ensuring reliable results.

The Core Problem: Reactivity with Lithium
The restriction against lithium is not a minor guideline; it is a fundamental chemical incompatibility that leads to electrode failure.
Why Lithium is Prohibited
Unlike surface fouling, which can often be cleaned, the interaction between lithium and platinum is a corrosive chemical reaction. This process permanently alters the platinum's structure and properties.
This means any experiment involving lithium ions, lithium metal, or lithium-containing materials cannot use standard platinum electrodes without risking their destruction.
The Consequences of Contact
When platinum is exposed to lithium, its surface and potentially its entire structure are degraded. This corrosion destroys the precise surface area and catalytic sites essential for electrochemical processes.
The damage renders the electrode unreliable for sensitive measurements and can introduce unwanted byproducts into your system, invalidating your results.
Beyond Lithium: Broader Operational Constraints
While lithium represents a specific chemical threat, effective use of platinum electrodes requires managing other environmental and physical factors.
Preventing Surface Contamination
Organic substances and other materials can "foul" the electrode surface. This process, known as passivation, involves contaminants adhering to the platinum and blocking its active sites.
Fouling reduces the electrode's efficiency and catalytic activity. Unlike lithium corrosion, this can sometimes be reversed with careful cleaning, but prevention is always the better course of action.
The Importance of Physical Integrity
Platinum electrodes, particularly in mesh or thin sheet form, are mechanically soft. They should not be subjected to impact, pressure, or bending.
Any mechanical damage alters the electrode's geometry and surface area. Since electrochemical measurements like current density are directly dependent on surface area, physical changes will introduce significant errors.
Understanding the Trade-offs of Platinum
Platinum is chosen for its perceived stability, but this stability has clear limits that must be respected.
The Assumption of Inertness
Platinum is exceptionally resistant to corrosion from a wide range of acids and other chemicals, which is why it is a preferred material for electrodes in demanding environments. This general inertness is its primary value proposition.
The Exception That Defines the Boundary
The reactivity with lithium is the most critical exception to platinum's general stability. It serves as a reminder that no material is universally inert. Knowing these specific chemical incompatibilities is crucial for any technical application.
Cost vs. Longevity
Platinum electrodes are a significant investment. Avoiding damage from prohibited substances like lithium, surface contaminants, and physical stress is not just about scientific accuracy—it is about protecting a valuable and sensitive asset.
Making the Right Choice for Your Goal
To ensure the integrity of your work and the longevity of your equipment, tailor your handling procedures to your specific application.
- If your primary focus is battery research or involves alkali metals: You must confirm that your electrolyte and materials are entirely free of lithium to prevent the immediate destruction of a platinum electrode.
- If your primary focus is general electrochemistry or analysis: Prioritize keeping the electrode surface pristine and free from organic residues or other contaminants that can foul the active sites and skew results.
- If you are setting up or handling the equipment: Always handle platinum electrodes with care, recognizing that their physical form is directly tied to their electrochemical performance.
Properly managing these chemical and physical constraints is the key to leveraging platinum's powerful capabilities while ensuring its long-term value.
Summary Table:
| Key Restriction | Primary Risk | Consequence |
|---|---|---|
| Contact with Lithium | Corrosive chemical reaction | Permanent electrode damage, unreliable data |
| Surface Contamination (Fouling) | Passivation from organics | Reduced catalytic activity, skewed results |
| Physical Damage (Bending/Impact) | Altered surface area | Measurement errors, loss of precision |
Protect your research and your investment. Platinum electrodes are a critical, high-value asset for precise electrochemical measurements. KINTEK specializes in lab equipment and consumables, serving laboratory needs with expert guidance on electrode selection, handling, and maintenance. Ensure your experiments are accurate and your equipment lasts longer—contact our experts today to discuss the right solutions for your specific application!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
People Also Ask
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements



















