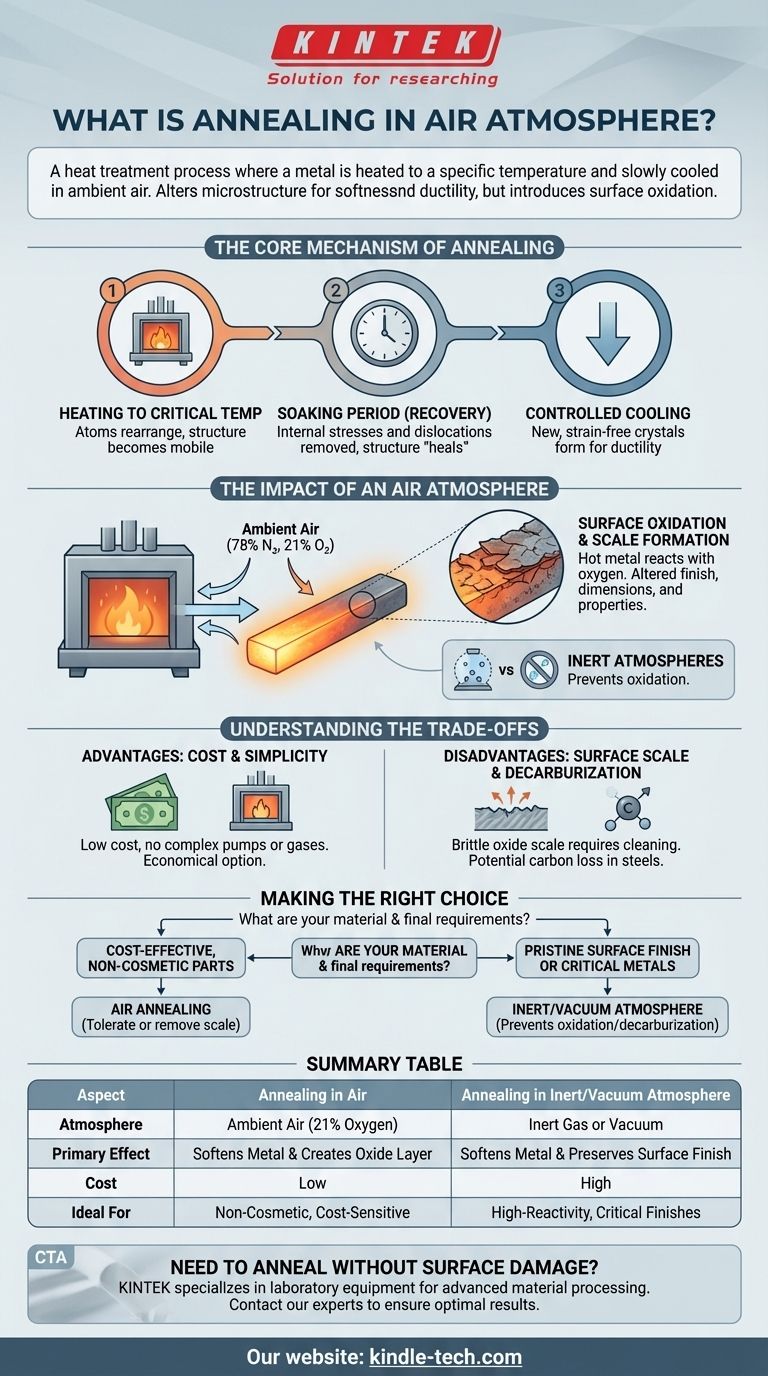
Annealing in an air atmosphere is a heat treatment process where a metal is heated to a specific temperature and slowly cooled in the presence of ambient air. This process alters the material's internal microstructure to make it softer, more ductile, and less brittle. The defining characteristic of this method is the interaction between the hot metal surface and the oxygen in the air, which leads to oxidation.
While annealing in air is the simplest and most cost-effective method to improve a metal's internal properties, it fundamentally introduces a trade-off: you gain the desired softness and ductility at the cost of creating an undesirable oxide layer on the material's surface.

The Core Mechanism of Annealing
Before considering the atmosphere, it's crucial to understand the fundamental stages of any annealing process. The goal is to repair the internal crystalline structure, which becomes strained and distorted during manufacturing processes like bending, rolling, or stamping.
Heating to a Critical Temperature
The metal is heated to a temperature below its melting point but high enough to allow its atoms to rearrange. This allows the internal crystalline structure to become more mobile, preparing it for repair.
The Soaking Period (Recovery)
The material is held at this elevated temperature for a set period. During this stage, the metal softens by removing internal stresses and linear defects known as dislocations. The a tomic structure essentially "heals" itself, leading to a more uniform and less stressed state.
Controlled Cooling
Finally, the metal is cooled at a very slow, controlled rate. This slow cooling is critical for allowing new, strain-free crystals (or grains) to form. The result is a more ductile and less brittle final product.
The Impact of an Air Atmosphere
The "atmosphere" is the gaseous environment surrounding the metal inside the furnace. Choosing to use simple ambient air has significant and predictable consequences.
What "Air Atmosphere" Means
This refers to performing the annealing process in normal air, which is composed of approximately 78% nitrogen and 21% oxygen. At the high temperatures required for annealing, the oxygen is highly reactive.
The Inevitable Reaction: Oxidation
The primary effect of annealing in air is oxidation. The hot surface of the metal reacts with the oxygen, forming a layer of metallic oxide. This layer is commonly known as scale or tarnish. This scale alters the surface finish, dimensions, and properties of the part.
The Alternative: Inert Atmospheres
To avoid oxidation, annealing can be performed in a controlled environment. This involves using a vacuum furnace to remove the air or flooding the furnace with an inert gas (like argon or nitrogen) that will not react with the metal.
Understanding the Trade-offs
The choice to anneal in air is almost always a decision based on balancing cost against final material requirements.
Advantage: Cost and Simplicity
Air is free, and the equipment required is simpler. Furnaces that operate with ambient air do not need expensive vacuum pumps, complex sealing systems, or a continuous supply of costly inert gas. This makes it the most economical heat treatment option.
Disadvantage: Surface Scale Formation
The oxide scale is the single biggest drawback. This layer is often brittle, flaky, and aesthetically undesirable. It almost always must be removed in a secondary operation, such as sandblasting, grinding, or chemical cleaning (acid pickling), which adds time and cost to the overall process.
Disadvantage: Potential for Decarburization
For carbon steels, annealing in an oxygen-rich atmosphere can cause another problem: decarburization. Oxygen can react with and remove carbon from the surface of the steel, leaving a softer outer layer that may compromise the part's intended hardness and wear resistance.
Making the Right Choice for Your Application
Selecting the right annealing atmosphere depends entirely on the material and the final requirements of the component.
- If your primary focus is cost-effectiveness for non-cosmetic parts: Air annealing is often the most practical choice, provided you can tolerate or later remove the resulting surface oxide layer.
- If your primary focus is maintaining a pristine surface finish: You must use a controlled atmosphere, such as a vacuum or an inert gas like argon, to prevent any oxidation.
- If you are working with high-carbon steels or highly reactive metals (like titanium): An inert or vacuum atmosphere is essential to prevent both oxidation and undesirable changes to the material's surface chemistry.
Ultimately, understanding the role of the atmosphere transforms annealing from a simple heating process into a precise engineering decision.
Summary Table:
| Aspect | Annealing in Air | Annealing in Inert/Vacuum Atmosphere |
|---|---|---|
| Atmosphere | Ambient Air (21% Oxygen) | Inert Gas (Argon/Nitrogen) or Vacuum |
| Primary Effect | Softens Metal & Creates Oxide Layer (Scale) | Softens Metal & Preserves Surface Finish |
| Cost | Low (Simple Equipment, No Gas) | High (Specialized Equipment, Gas/Vacuum) |
| Ideal For | Non-Cosmetic Parts, Cost-Sensitive Applications | High-Reactivity Metals, Critical Surface Finishes |
Need to Anneal a Critical Component Without Surface Damage?
Choosing the right heat treatment process is crucial for your material's performance. While air annealing is cost-effective, it may not be suitable for parts requiring pristine surfaces or precise material properties.
KINTEK specializes in laboratory equipment and consumables for advanced material processing. We can help you select the right furnace and atmosphere control system for your specific annealing needs, whether you're working with standard steels or highly reactive metals.
Contact our experts today to discuss your application and ensure optimal results for your laboratory work.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vertical Laboratory Tube Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- What is an inert condition? A Guide to Preventing Fires and Explosions
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting



















