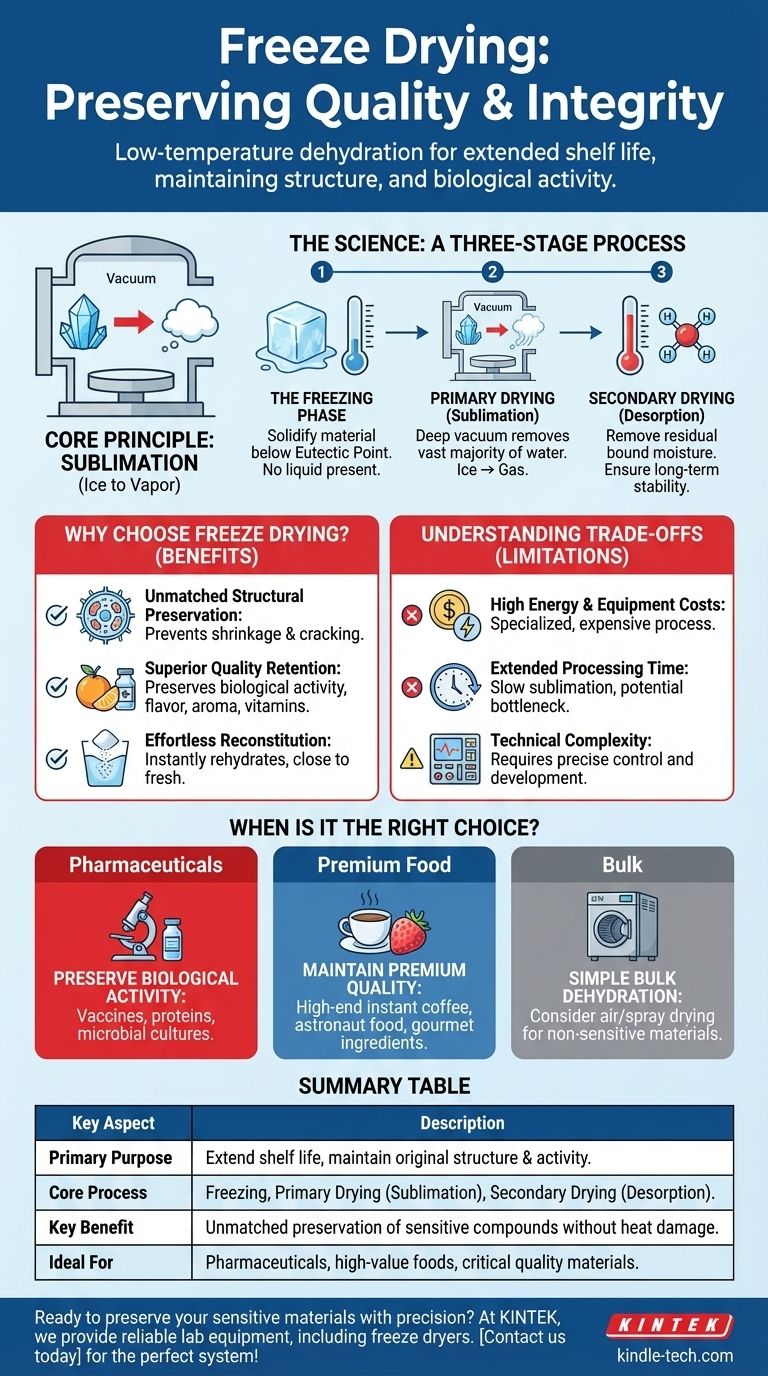
In essence, freeze drying is a low-temperature dehydration process that removes moisture from a product to preserve it. Also known as lyophilization, its primary purpose is to extend the shelf life of perishable materials while meticulously maintaining their original structure, quality, and biological activity.
The core principle of freeze drying is its ability to gently remove water by transforming ice directly into vapor, a process called sublimation. This bypasses the liquid water stage, which is often responsible for the damage and degradation seen in traditional drying methods.

The Science of Freeze Drying: A Three-Stage Process
Understanding freeze drying requires looking at its distinct, sequential stages. Each step is precisely controlled to ensure the final product is preserved with the highest possible fidelity.
Stage 1: The Freezing Phase
The first step is to thoroughly freeze the material. This is a critical stage that determines the final structure of the product.
The goal is to bring the material well below its eutectic point, which is the lowest temperature at which the product can exist only in a solid phase. This ensures no liquid is present before the drying process begins.
Stage 2: Primary Drying (Sublimation)
This is the heart of the freeze-drying process. After freezing, the material is placed under a deep vacuum.
This reduction in pressure allows the frozen water to change directly from a solid (ice) to a gas (water vapor) without ever becoming a liquid. This sublimation process removes the vast majority of the water content.
Stage 3: Secondary Drying (Desorption)
Even after primary drying, some water molecules remain bound to the material.
In this final stage, the temperature is gradually raised (while still under vacuum) to break these bonds and remove the remaining residual moisture. This step is crucial for ensuring long-term stability.
Why Choose Freeze Drying Over Other Methods?
While other dehydration methods exist, freeze drying is chosen when the integrity of the original product is paramount. Its benefits stem directly from avoiding the destructive nature of liquid water and high heat.
Unmatched Structural Preservation
Because water is removed from a solid state, the material's underlying physical structure is left intact. This prevents the shrinkage, cracking, and collapse common with heat-based dehydration.
Superior Quality Retention
The low-temperature process has a minimal impact on sensitive compounds. This means it excels at preserving biological activity (in vaccines or proteins), vitamins, flavor, and aroma in food products.
Effortless Reconstitution
Freeze-dried products are highly porous, allowing them to rehydrate almost instantly and completely when water is reintroduced. The final reconstituted product is considered exceptionally close to its fresh counterpart in quality.
Understanding the Trade-offs
Despite its significant advantages, freeze drying is not a universal solution. The precision that makes it so effective also introduces practical limitations that must be considered.
High Energy and Equipment Costs
Achieving the necessary low temperatures and deep vacuums requires specialized, energy-intensive equipment. This makes freeze drying a significantly more expensive process compared to simple air or heat drying.
Extended Processing Time
The multi-stage process, particularly the slow sublimation phase, can take many hours or even days to complete. This long cycle time can be a bottleneck in high-volume production environments.
Technical Complexity
Successfully freeze drying a product requires careful development and precise control over temperature and pressure. It is a technically demanding process best suited for high-value materials where quality preservation justifies the investment.
When is Freeze Drying the Right Choice?
Choosing this method depends entirely on your preservation goals and the nature of your product.
- If your primary focus is preserving biological activity: Freeze drying is the industry standard for stabilizing vaccines, pharmaceuticals, proteins, and microbial cultures.
- If your primary focus is maintaining premium food quality: It is the ideal choice for high-end instant coffee, astronaut food, and gourmet ingredients where taste, texture, and nutritional value must be preserved.
- If your primary focus is simple bulk dehydration: For materials that are not sensitive to heat or structural changes, less expensive methods like air drying or spray drying are often more practical.
Ultimately, freeze drying is an investment in unparalleled quality and stability for your most sensitive materials.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Purpose | Extend shelf life while maintaining original structure, quality, and biological activity. |
| Core Process | Three-stage dehydration: Freezing, Primary Drying (sublimation), Secondary Drying (desorption). |
| Key Benefit | Unmatched preservation of sensitive compounds, flavors, and textures without heat damage. |
| Ideal For | Pharmaceuticals, high-value foods, and materials where quality retention is critical. |
Ready to preserve your sensitive materials with precision?
At KINTEK, we specialize in providing reliable lab equipment, including freeze dryers, to help you achieve superior results in pharmaceuticals, food science, and biotechnology. Our solutions ensure your products maintain their integrity, activity, and quality throughout the preservation process.
Contact us today to find the perfect freeze-drying system for your laboratory's unique needs!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Lab-Scale Vacuum Induction Melting Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Vibratory Sieve Shaker Machine Dry Three-Dimensional Vibrating Sieve
People Also Ask
- Why is freeze drying important for certain chemical products? Preserve Integrity & Extend Shelf Life
- What are the main uses of laboratory freeze dryers? Preserve Sensitive Materials with Precision Lyophilization
- What is the eutectic point in freeze drying? The Critical Temperature for Successful Lyophilization
- Why is a Laboratory Freeze Dryer essential? Preserve Sample Integrity for Long-Term Stability
- What are some do's and don'ts when using a Laboratory Freeze Dryer? Master the Core Principles for Success



















