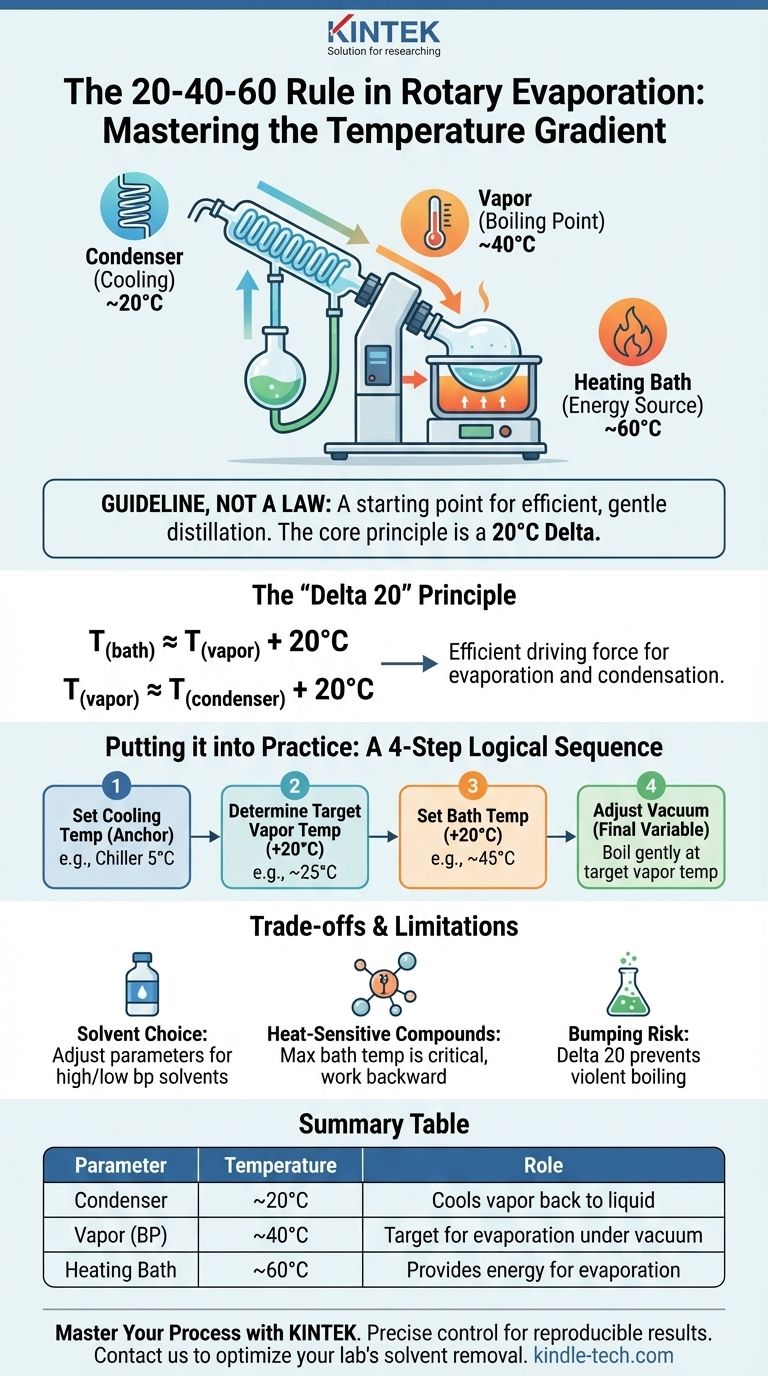
The 20-40-60 rule is a widely cited guideline for setting up a rotary evaporator (rotovap). It provides a starting point for the target temperatures across the system to achieve efficient distillation: 20°C for the condenser, a resulting 40°C vapor temperature for the boiling solvent, and 60°C for the heating bath. This rule is a practical application of a more fundamental principle that ensures a proper temperature gradient for controlled solvent removal.
The "20-40-60" rule is less about three fixed numbers and more about creating an optimal temperature gradient. The core principle is to maintain a 20°C difference between the heating bath, the solvent's boiling point, and the condenser to create a powerful and efficient driving force for distillation.

Deconstructing the "Why" Behind the Rule
To use a rotovap effectively, you must understand the physics at play. The 20-40-60 rule is simply a convenient memory aid for applying these physical principles.
The Goal: Gentle Evaporation Under Vacuum
A rotary evaporator's primary function is to gently remove a solvent from a sample. It does this by lowering the pressure inside the system with a vacuum pump.
Lowering the pressure dramatically reduces the solvent's boiling point. This allows you to evaporate solvents like water or ethanol at a mild 40°C instead of their standard 100°C or 78°C boiling points, protecting your sample from heat-related degradation.
The Role of the Temperature Gradient
Efficient operation relies on a specific flow of energy, managed by a temperature gradient. The 20-40-60 rule creates this gradient.
- The Bath (60°C): Provides the energy needed to turn the liquid solvent into a gas (vapor).
- The Vapor (40°C): The solvent boils at this temperature due to the reduced pressure.
- The Condenser (20°C): This cold surface removes energy from the vapor, causing it to condense back into a liquid and collect in the receiving flask.
This steady, downward temperature slope ensures the solvent moves efficiently from the evaporating flask to the receiving flask.
The "Delta 20" Principle
The 20-40-60 rule is one specific example of the more universal "Delta 20" principle. This principle states that for ideal efficiency, you should aim for a 20°C difference between each stage of the process.
T(bath) ≈ T(vapor) + 20°CT(vapor) ≈ T(condenser) + 20°C
This 20°C gap provides a strong but manageable driving force for both evaporation and condensation, preventing violent bumping while ensuring a rapid distillation rate.
Putting the Principle into Practice
Instead of blindly setting the numbers to 20, 40, and 60, think of the process as a logical sequence based on the "Delta 20" principle.
Step 1: Set Your Cooling Temperature
Your condenser temperature is the anchor for the entire system. This is determined by your equipment. Tap water might be 15-20°C, while a dedicated chiller can reliably hold 0-5°C. This is your starting point.
Step 2: Determine Your Target Vapor Temperature
Your target vapor temperature (the solvent's boiling point under vacuum) should be approximately 20°C warmer than your condenser. If your chiller is set to 5°C, you should aim for a vapor temperature of around 25°C.
Step 3: Set Your Bath Temperature
The heating bath must provide enough energy to get the solvent to its target vapor temperature. Following the principle, you should set the bath about 20°C warmer than your target vapor temperature. For a 25°C vapor target, a 45°C bath is an ideal starting point.
Step 4: Adjust the Vacuum
The vacuum level is the final variable you adjust to hit your target. After setting your temperatures, slowly decrease the pressure until your solvent begins to boil gently and the thermometer on the rotovap registers your target vapor temperature (e.g., 25°C). You can use a vacuum nomograph to find a good starting pressure for your specific solvent.
Understanding the Trade-offs and Limitations
The 20-40-60 rule is an excellent starting point, but it is not a universal law. Applying it without critical thought can be inefficient or even risk your sample.
It's a Guideline, Not a Law
Always treat these numbers as a starting point. Your specific solvent, sample stability, and equipment performance will require fine-tuning.
The Impact of Solvent Choice
High-boiling-point solvents like water or DMSO require a much stronger vacuum or a higher bath temperature to achieve evaporation compared to low-boiling-point solvents like dichloromethane or ethyl acetate. You must adjust your parameters accordingly.
Protecting Heat-Sensitive Compounds
This is the most critical exception. If your compound degrades above 30°C, that is your absolute maximum bath temperature. You must work backward from there, using a lower vapor temperature and a deeper vacuum to compensate. The safety of your sample always overrides the speed of evaporation.
The Risk of Bumping and Foaming
If the temperature difference between the bath and the solvent's boiling point is too large, the boiling can become explosive. This "bumping" can splash your sample into the condenser, leading to loss of material. The "Delta 20" rule helps maintain a controlled, gentle boil.
How to Apply This to Your Experiment
Use this principle as a logical framework, not a rigid command, to set up your experiment correctly.
- If your primary focus is speed with a robust sample: The standard 60°C bath temperature is an excellent starting point for common solvents like ethanol or water, assuming your condenser is sufficiently cool.
- If your primary focus is protecting a heat-sensitive compound: Start with the maximum safe bath temperature (e.g., 30°C) and adjust your vacuum and cooling to achieve a gentle boil at a vapor temperature of around 10-15°C.
- If you are using a powerful chiller (e.g., 0-5°C): You can achieve high efficiency with a much lower bath temperature (e.g., 40°C), which is safer for nearly all compounds.
Mastering the temperature gradient, not just memorizing numbers, is the key to efficient and reliable rotary evaporation.
Summary Table:
| Parameter | Temperature | Role in Distillation |
|---|---|---|
| Condenser | ~20°C | Cools vapor back to liquid for collection |
| Vapor (Solvent Boiling Point) | ~40°C | Target temperature for solvent evaporation under vacuum |
| Heating Bath | ~60°C | Provides energy to evaporate the solvent |
Master Your Rotary Evaporation Process with KINTEK
Are you looking to optimize your lab's solvent removal for better efficiency and sample protection? The principles behind the 20-40-60 rule are key to achieving gentle, controlled evaporation.
At KINTEK, we specialize in high-quality laboratory equipment, including reliable rotary evaporators and efficient chilling systems that give you precise control over these critical temperature gradients. Whether you are processing robust samples or highly sensitive compounds, the right equipment is essential for reproducible results.
Let our experts help you select the perfect rotovap system for your specific needs. Contact our team today to discuss how we can enhance your laboratory's capabilities and ensure your distillation processes are both safe and effective.
Visual Guide
Related Products
- Evaporation Crucible for Organic Matter
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
- Rotating Platinum Disk Electrode for Electrochemical Applications
People Also Ask
- How long does it take for THC to evaporate? The Real Science Behind Potency Loss
- Do cannabinoids evaporate? How to Preserve Potency and Prevent Degradation
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What precautions should be taken in a chemistry lab? Master the RAMP Framework for Ultimate Safety
- What are the 5 factors that affect the rate of evaporation? Master the Process for Your Lab



















