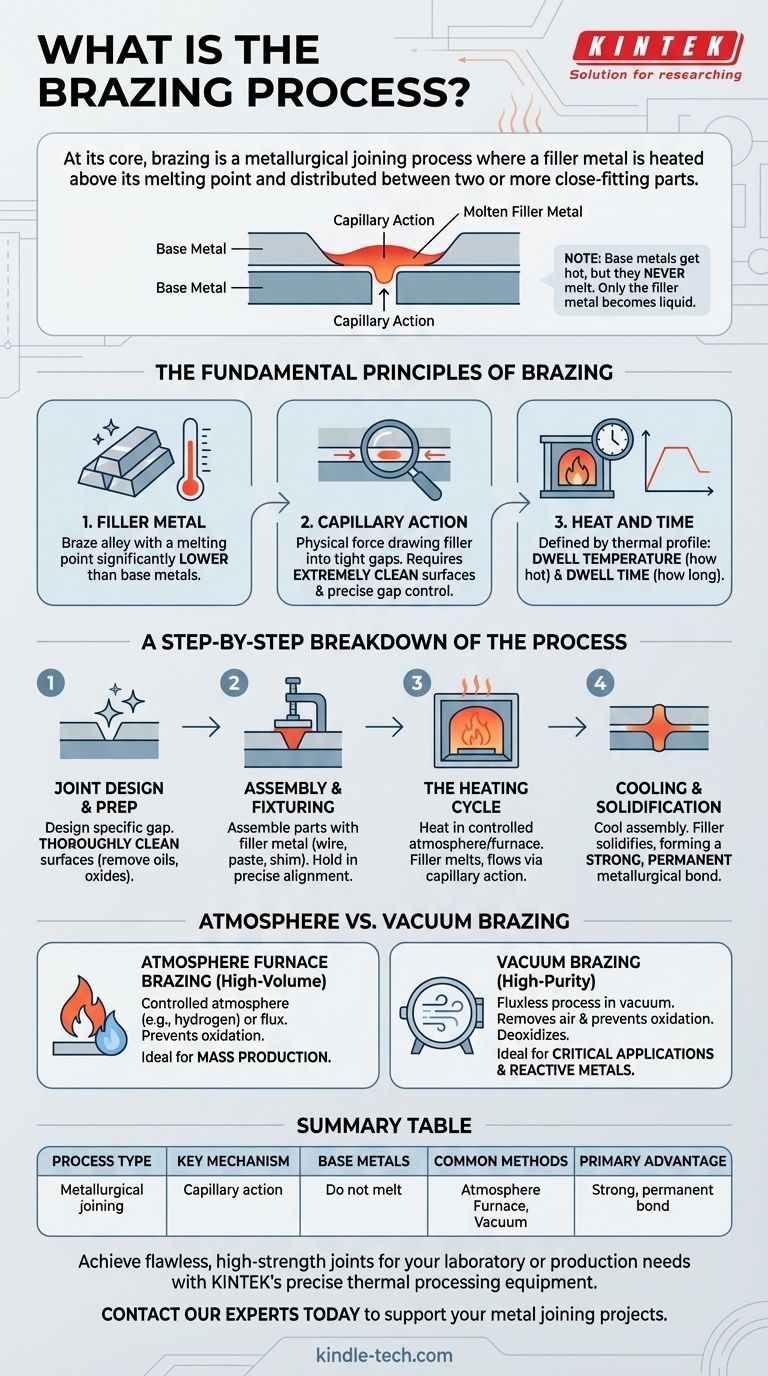
At its core, brazing is a metallurgical joining process where a filler metal is heated above its melting point and distributed between two or more close-fitting parts. The filler metal, which has a lower melting point than the base metals being joined, is drawn into the gap via capillary action. As the assembly cools, the filler solidifies to form a strong, permanent metallurgical bond without melting the base materials.
Brazing is not simply "gluing with metal." It is a precise thermal process that relies on creating chemically clean surfaces and controlling temperature to allow a molten filler alloy to wet and flow into a joint, creating a bond that is often as strong as the parent metals themselves.

The Fundamental Principles of Brazing
To master brazing, one must understand the three core elements that make it work: the filler metal, the capillary action, and the controlled heating environment. These principles are universal, whether you are joining simple copper tubes or complex aerospace components.
The Role of the Filler Metal
The filler metal, or braze alloy, is the heart of the joint. Its composition is chosen so that its melting point is significantly lower than that of the base metals being joined.
This distinction is critical: the base metals get hot, but they never melt. Only the filler metal becomes liquid.
The Importance of Capillary Action
Capillary action is the physical force that draws the liquid filler metal into the tight gap between the parts. For this to work, two conditions are essential.
First, the parts must be extremely clean and free of oxides. Second, the gap between the parts must be precisely controlled—neither too wide nor too narrow.
The Critical Parameters: Heat and Time
Every brazing operation is defined by its thermal profile. The key parameters are the dwell temperature (how hot it gets) and the dwell time (how long it stays hot).
The goal is to heat the entire assembly uniformly to a temperature that is above the filler's melting point but below the base metals' melting points, holding it just long enough for the filler to flow completely through the joint.
A Step-by-Step Breakdown of the Process
While specific techniques vary, the fundamental sequence of furnace brazing provides a clear model for understanding the process from start to finish.
Step 1: Joint Design and Preparation
Success begins before the parts ever see heat. The joint must be designed with a specific gap to promote capillary action.
Crucially, the surfaces of the base metals must be thoroughly cleaned to remove any oils, dirt, and oxide layers that would prevent the filler metal from wetting the surface.
Step 2: Assembly and Fixturing
The cleaned parts are assembled, often with the filler metal pre-placed in or near the joint in the form of a wire, paste, or shim.
The assembly is held in precise alignment using clamps or support fixtures to ensure it does not move during the heating and cooling cycle.
Step 3: The Heating Cycle
The entire assembly is heated in a controlled atmosphere, most commonly a furnace. As the temperature rises, any residual oxides are often broken down by thermal expansion or removed by flux or the furnace environment itself.
Once the assembly reaches the target temperature, the filler metal melts and is pulled through the entire joint by capillary action.
Step 4: Cooling and Solidification
After the filler has fully penetrated the joint, the assembly is cooled in a controlled manner. As it cools, the filler metal solidifies, creating a solid, continuous metallurgical bond between the parts. The finished assembly is then cleaned if necessary.
Understanding the Trade-offs: Atmosphere vs. Vacuum
The environment in which brazing occurs has a massive impact on the final joint quality. The two most common industrial methods are atmosphere furnace brazing and vacuum brazing.
Furnace Brazing: The High-Volume Workhorse
In standard furnace brazing, the heating chamber is filled with a controlled atmosphere (like hydrogen) or a flux is used. This prevents the base metals from oxidizing at high temperatures.
This method is exceptionally efficient for creating thousands of joints simultaneously, making it ideal for mass production. However, using flux may require a post-braze cleaning step to remove corrosive residues.
Vacuum Brazing: The High-Purity Specialist
Vacuum brazing is a fluxless process performed in a low-pressure chamber (a vacuum). Instead of relying on a gas or chemical flux, the vacuum itself removes air and prevents oxidation.
This high-purity environment can even pull contaminants and oxides out of the metal, a process known as "deoxidizing." The result is an exceptionally strong, clean joint, making it the preferred method for critical applications and reactive metals.
Making the Right Choice for Your Application
Selecting the correct brazing method depends entirely on your project's goals for volume, material, and final quality.
- If your primary focus is high-volume production of non-reactive metals: Standard furnace brazing offers unmatched efficiency for creating strong, reliable joints at scale.
- If your primary focus is joining reactive metals or achieving maximum joint purity: Vacuum brazing is the superior choice as it eliminates the need for flux and prevents oxidation at the most fundamental level.
- If your primary focus is a successful joint regardless of method: Meticulous surface cleaning and precise joint gap control are the most critical factors for success.
By understanding these core principles, you can leverage brazing to create robust, reliable joints for even the most demanding applications.
Summary Table:
| Brazing Aspect | Key Detail |
|---|---|
| Process Type | Metallurgical joining |
| Key Mechanism | Capillary action |
| Base Metals | Do not melt |
| Common Methods | Atmosphere Furnace, Vacuum |
| Primary Advantage | Strong, permanent bond |
Achieve flawless, high-strength joints for your laboratory or production needs. KINTEK specializes in the precise thermal processing equipment essential for successful brazing operations. Whether you require the high-volume efficiency of atmosphere furnaces or the high-purity results of vacuum brazing systems, our expertise in lab equipment ensures you have the right tool for your specific materials and quality demands. Contact our experts today to discuss how we can support your metal joining projects.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- What is the difference between annealing and quenching? Master Heat Treatment for Optimal Material Properties
- What is the process of quenching? A Guide to Controlled Metal Hardening
- What are the different types of annealing furnace? A Guide to Choosing the Right System for Your Needs
- What is the function of vacuum drying equipment in Li6PS5Cl composite preparation? Ensure High Ionic Conductivity
- What PPE should you ensure is available when operating a furnace? A Complete Guide to Staying Safe
- What changes in the annealing process? A Guide to the 3 Key Microstructural Stages
- How does a vacuum drying oven benefit PANI post-treatment? Preserve Conductivity and Structural Integrity
- What role do furnaces play in argyrodite electrolytes? Essential Tools for High-Performance Phase Formation



















