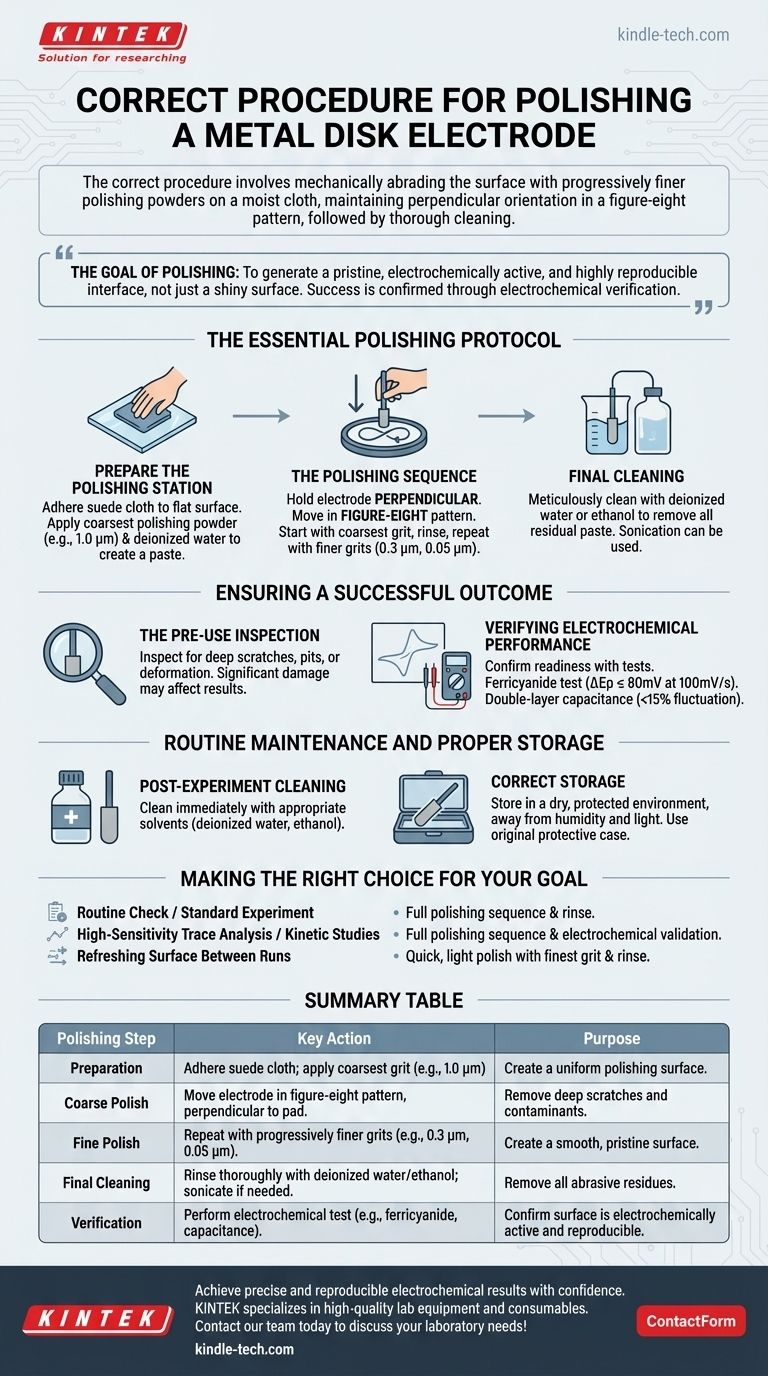
The correct procedure for polishing a metal disk electrode involves mechanically abrading its surface with a sequence of progressively finer polishing powders on a moist cloth. The key is to maintain a perfectly perpendicular orientation while moving the electrode in a figure-eight pattern. This process is followed by a thorough cleaning to remove all abrasive particles, ensuring a clean and reproducible surface for electrochemical experiments.
The goal of polishing is not merely to create a shiny surface, but to generate a pristine, electrochemically active, and highly reproducible interface. The mechanical polishing steps are only the means to an end; the true measure of success is confirmed through electrochemical verification.

The Essential Polishing Protocol
A successful polish relies on a methodical approach, moving from coarse to fine abrasives to systematically remove surface damage and contaminants.
Step 1: Prepare the Polishing Station
First, adhere a suede polishing cloth to a flat, stable surface like a glass polishing plate. This provides a uniform base for the procedure.
Apply a small amount of the coarsest polishing powder (e.g., 1.0 µm alumina or diamond) to the cloth. Add a few drops of deionized or distilled water to create a thin, consistent paste.
Step 2: The Polishing Sequence
Hold the electrode with the disk facing the cloth, ensuring it is perfectly perpendicular to the polishing pad. This vertical orientation is critical to prevent rounding the electrode's edges, which would alter its effective surface area.
Gently press down and move the electrode in a figure-eight or circular motion. This pattern ensures the surface is abraded evenly from all directions.
Continue polishing with the coarsest grit until all visible scratches are removed. Then, clean the electrode thoroughly with deionized water before moving to the next step.
Repeat this entire process with progressively finer polishing powders, such as 0.3 µm and finally 0.05 µm. Each new step should only be started after the electrode has been rinsed clean of the previous, larger grit.
Step 3: Final Cleaning
Once the final polishing step with the finest powder (0.05 µm) is complete, the electrode must be meticulously cleaned.
Thoroughly rinse it with deionized water or ethanol to remove all residual polishing paste. Sonication in deionized water can be used for a more rigorous cleaning if necessary.
Ensuring a Successful Outcome: Inspection and Verification
A visually clean electrode is not a guarantee of electrochemical viability. You must inspect it before polishing and verify its performance afterward.
The Pre-Use Inspection
Before you even begin, carefully inspect the electrode surface. Look for deep scratches, pits, or physical deformation.
Significant damage cannot always be fixed by polishing and may negatively affect the accuracy of your results by altering mass transport and electron transfer at the surface.
Verifying Electrochemical Performance
After polishing and cleaning, you can confirm the electrode's readiness with electrochemical tests. This is the ultimate check of your procedure.
One standard method is a potassium ferricyanide test. In a cyclic voltammetry experiment, a well-polished electrode should exhibit a peak potential separation (ΔEp) of less than or equal to 80mV at a 100mV/s scan rate.
Another method is a double-layer capacitance measurement. When tested in a 0.1M KCl solution, the capacitance fluctuation should remain below 15%, indicating a clean and stable surface.
Routine Maintenance and Proper Storage
Proper care extends the life of your electrode and ensures reliable performance over time.
Post-Experiment Cleaning
Immediately after an experiment, remove the electrode from the cell. Clean it with appropriate solvents, such as deionized water or ethanol, to remove any remaining electrolyte or impurities.
Correct Storage
After cleaning, dry the electrode completely. Store it in a dry, protected environment, away from humidity, high temperatures, and strong light.
Using the electrode's original protective case is the best practice for preventing accidental damage.
Making the Right Choice for Your Goal
The rigor of your polishing and verification should match the demands of your experiment.
- If your primary focus is a routine check or standard experiment: A full polishing sequence (1.0 µm -> 0.3 µm -> 0.05 µm) followed by a thorough rinse is your standard procedure.
- If your primary focus is high-sensitivity trace analysis or kinetic studies: You must perform the full polishing sequence and then validate the surface using an electrochemical test like the ferricyanide standard.
- If your primary focus is refreshing the surface between repetitive runs: A quick, light polish with only the finest grit (0.05 µm) followed by rinsing can be sufficient to restore performance.
Ultimately, a well-prepared electrode is the foundation upon which accurate and reproducible electrochemical data is built.
Summary Table:
| Polishing Step | Key Action | Purpose |
|---|---|---|
| Preparation | Adhere suede cloth; apply coarsest grit (e.g., 1.0 µm) | Create a uniform polishing surface. |
| Coarse Polish | Move electrode in figure-eight pattern, perpendicular to pad. | Remove deep scratches and contaminants. |
| Fine Polish | Repeat with progressively finer grits (e.g., 0.3 µm, 0.05 µm). | Create a smooth, pristine surface. |
| Final Cleaning | Rinse thoroughly with deionized water/ethanol; sonicate if needed. | Remove all abrasive residues. |
| Verification | Perform electrochemical test (e.g., ferricyanide, capacitance). | Confirm surface is electrochemically active and reproducible. |
Achieve precise and reproducible electrochemical results with confidence. The correct electrode preparation is critical for the success of your experiments. KINTEK specializes in high-quality lab equipment and consumables, including the precise polishing powders and cloths needed for this procedure. Let our experts help you select the right materials to ensure your electrode surfaces are perfectly prepared for your specific application, from routine analysis to high-sensitivity studies. Contact our team today to discuss your laboratory needs and enhance your data quality!
Visual Guide
Related Products
- Platinum Auxiliary Electrode for Laboratory Use
- Electrolytic Electrochemical Cell for Coating Evaluation
- Warm Isostatic Press for Solid State Battery Research
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
People Also Ask
- Why is a platinum electrode typically selected as the auxiliary or counter electrode? Unlock Precise Data Accuracy
- What is the benefit of using a platinized platinum wire as a counter electrode? Optimize Operando Study Precision
- Why is platinum a good counter electrode? For Superior Chemical Inertness and Electron Transfer
- What are the advantages of using a Platinum (Pt) electrode for zirconium testing? Ensure High-Precision Data Integrity
- What is the function of a platinum electrode as an auxiliary electrode? Ensure Precise Nickel Coating Corrosion Testing



















