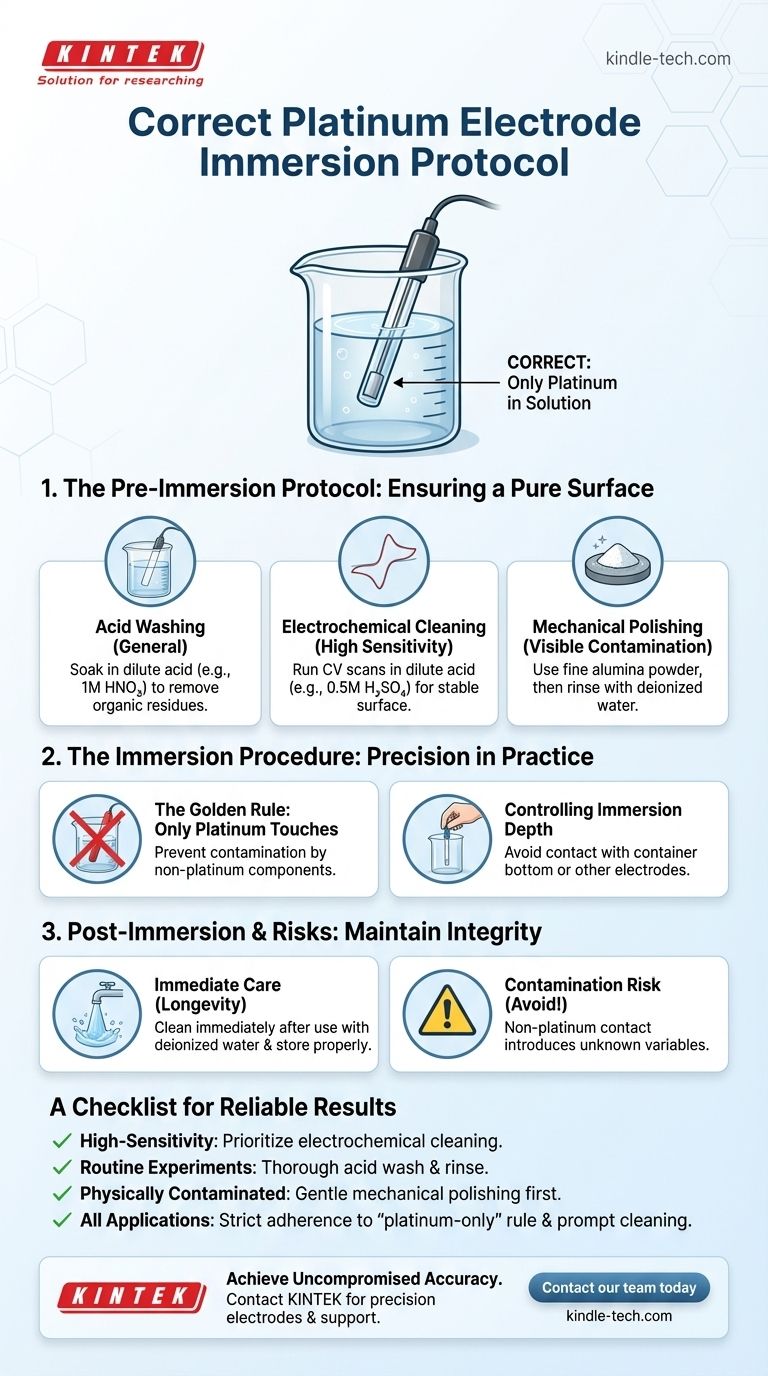
The correct immersion of a platinum electrode is a procedure demanding precision. You must submerge only the platinum part of the electrode into the electrolyte solution. All other components, such as the casing or connecting wire, must remain strictly out of the liquid to prevent contamination and ensure accurate results.
The integrity of your electrochemical measurement hinges on more than just the moment of immersion. It is a disciplined, three-part process: meticulous cleaning before use, controlled positioning during the experiment, and immediate care afterward.

The Pre-Immersion Protocol: Ensuring a Pure Surface
The state of the electrode's surface directly dictates the quality of your data. Any impurities, oxides, or contaminants can alter the electrochemical reactions, leading to inaccurate and non-repeatable results.
The Importance of a Clean Surface
A pristine platinum surface provides a known, consistent interface for the electrochemical reaction. The goal of cleaning is to remove any adsorbed species or oxide layers from previous use or storage, restoring the electrode to a baseline state.
Method 1: Acid Washing
For general-purpose cleaning, a simple acid wash is effective. Soak the platinum tip in a dilute acid solution, such as 1M nitric acid (HNO₃), for several minutes. This removes most organic residues and metallic impurities.
Method 2: Electrochemical Cleaning
For more rigorous applications requiring a highly active and reproducible surface, electrochemical cleaning is the standard. This typically involves running cyclic voltammetry scans in a dilute sulfuric acid (e.g., 0.5M H₂SO₄) solution until a stable, characteristic voltammogram for platinum is obtained.
Method 3: Mechanical Polishing
If the electrode surface is visibly scratched, tarnished, or has stubborn contamination, mechanical polishing may be necessary. Use a fine polishing powder like 0.05 micron alumina on a polishing pad, followed by thorough rinsing with deionized water to remove all abrasive particles.
The Immersion Procedure: Precision in Practice
Proper physical handling during the experiment is just as critical as the chemical preparation. Platinum wire and rod electrodes are delicate instruments.
The Golden Rule: Only Platinum Touches the Electrolyte
This is the most critical rule. Any contact between the electrolyte and the electrode's non-platinum body (often PTFE or glass) can leach impurities into your solution, creating unintended side reactions and invalidating your measurements.
Controlling Immersion Depth
Carefully lower the electrode into the solution, ensuring the platinum is sufficiently submerged but does not touch the bottom of the container or any other electrodes. Contact can cause a short circuit or introduce physical interference that disrupts the measurement.
Handling with Care
Platinum electrodes are fragile and expensive. Always handle them gently to avoid bending the wire or scratching the surface, which can permanently alter its electrochemical behavior.
Understanding the Trade-offs and Risks
Failing to follow the correct procedure introduces significant variables that compromise your work.
The Risk of Contamination
Immersing the non-platinum body is the most common mistake. It introduces unknown variables, making it impossible to attribute the measured response solely to the intended reaction at the platinum surface.
The Danger of Corrosion
While highly resistant, platinum is not completely inert under all conditions. Be certain that your chosen electrolyte and experimental parameters are not overly corrosive, as this can permanently damage the electrode surface.
The Impact of Improper Cleaning
Inadequate cleaning leads to poor reproducibility. Conversely, overly aggressive polishing can alter the surface roughness and effective surface area of the electrode, changing its response characteristics over time.
A Checklist for Reliable Results
Your approach should be tailored to the demands of your experiment. Use this checklist to ensure the integrity of your work and the longevity of your equipment.
- If your primary focus is high-sensitivity analytical accuracy: Prioritize electrochemical cleaning until a stable voltammogram is achieved, as this ensures the most active and repeatable surface.
- If you are performing routine experiments: A thorough acid wash followed by a deionized water rinse is a sufficient and reliable daily protocol.
- If your electrode is physically contaminated or performing poorly: Begin with gentle mechanical polishing before proceeding with chemical and electrochemical cleaning methods.
- For all applications: Strict adherence to the "platinum-only" immersion rule and prompt post-experiment cleaning are non-negotiable for maintaining data quality.
Following these disciplined steps ensures not only the accuracy of your results but also the longevity of this critical and valuable tool.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1. Pre-Immersion | Clean the platinum surface (acid wash, electrochemical cleaning, or polishing). | Remove contaminants and oxides for a consistent, active surface. |
| 2. During Immersion | Submerge only the platinum part; avoid contact with container or other electrodes. | Prevent contamination, short circuits, and physical damage. |
| 3. Post-Immersion | Clean immediately after use and store properly. | Maintain electrode longevity and performance for future experiments. |
Achieve Uncompromised Accuracy in Your Electrochemical Measurements
Proper electrode handling is fundamental to reliable data. KINTEK specializes in high-quality lab equipment and consumables, including precision electrodes, to support your research integrity.
Let our experts help you select the right tools and optimize your protocols. Contact our team today for a consultation and ensure your laboratory's success with equipment you can trust.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis
- What is the RRDE in electrochemistry? Unlock Detailed Reaction Pathways with Dual-Electrode Analysis
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What is the difference between ring disk electrode and rotating disk electrode? Unlock Deeper Electrochemical Insights



















