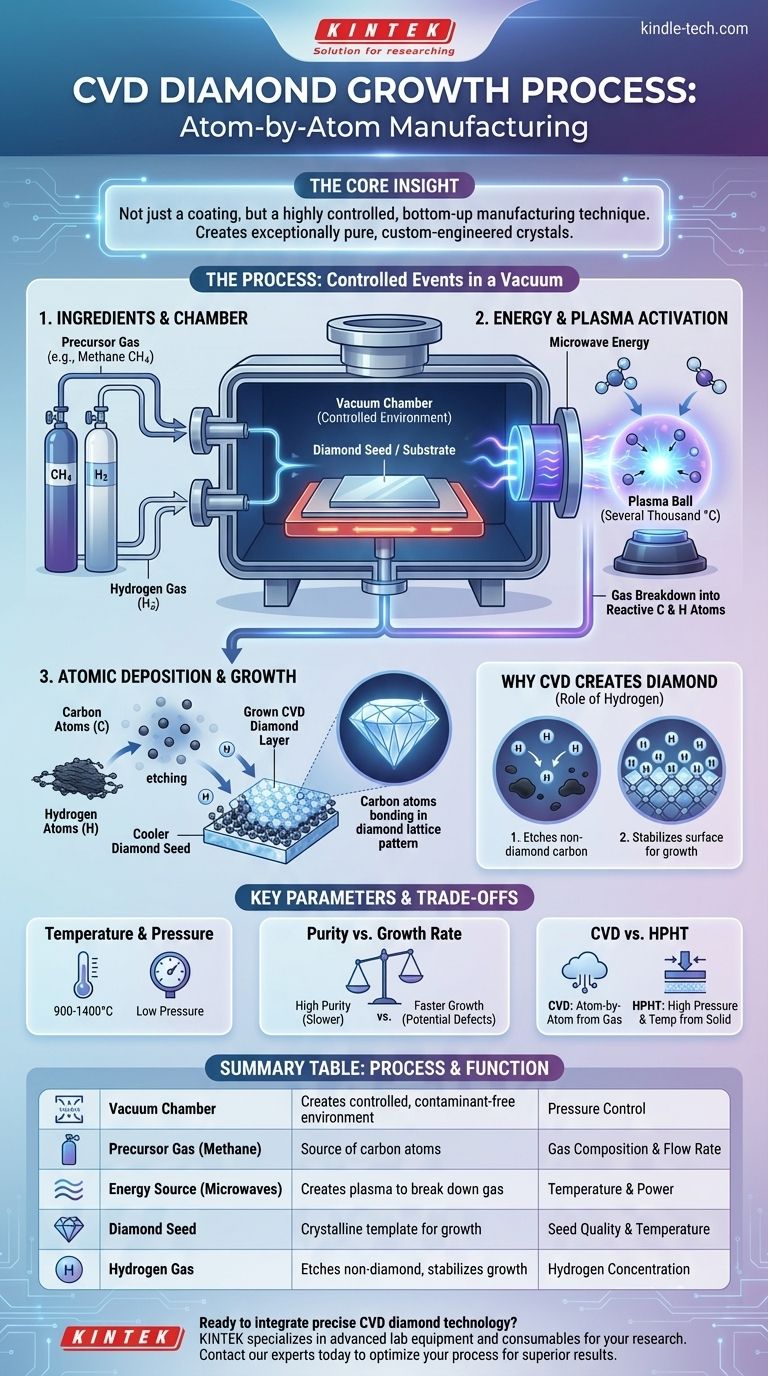
In essence, the Chemical Vapor Deposition (CVD) diamond growth process is a method for building a diamond atom by atom. It involves introducing a carbon-rich gas into a vacuum chamber, using energy to break that gas down into its fundamental carbon atoms, and allowing those atoms to settle onto a substrate, or "seed," where they form a new diamond layer. This technique effectively grows a diamond from a gaseous state.
The core insight is that CVD is not just a coating process; it is a highly controlled, bottom-up manufacturing technique. By precisely managing gas, temperature, and pressure, scientists can dictate the atomic structure of the material as it forms, allowing them to create exceptionally pure, custom-engineered diamond crystals.

The Core Principles of CVD Growth
To understand the CVD process, it's best to visualize it as a sequence of controlled events happening inside a specialized reactor. Each step is critical for ensuring the carbon atoms assemble into a diamond lattice rather than another form of carbon, like graphite.
The Chamber: A Controlled Vacuum Environment
The entire process takes place within a sealed vacuum chamber. This allows for precise control over pressure and prevents contamination from atmospheric gases like nitrogen and oxygen, which would interfere with the chemical reactions.
The Ingredients: Precursor Gas and the Diamond Seed
A carefully formulated mixture of gases is pumped into the chamber. For diamond growth, this is typically a precursor gas like methane (CH4), which provides the source of carbon, mixed with a much larger volume of hydrogen (H2).
A small, flat plate of existing diamond, known as a substrate or seed, is placed inside the chamber. This seed provides the crystalline template upon which the new diamond will grow.
The Catalyst: Activating the Gas with Energy
The chamber is filled with energy, usually in the form of microwaves, which generates a superheated ball of plasma. This intense energy, reaching temperatures of several thousand degrees Celsius, breaks apart the methane and hydrogen molecules into a cloud of reactive carbon and hydrogen atoms.
The Growth: Atomic Layer-by-Layer Deposition
This cloud of atoms moves towards the cooler diamond seed. Carbon atoms from the dissociated methane gas then deposit onto the seed's surface.
Because the seed has a diamond crystal structure, it acts as a template, guiding the new carbon atoms to bond in the exact same diamond lattice pattern. Over many hours or days, this atomic deposition builds up, layer by layer, growing a larger, pure diamond crystal.
Why CVD Creates Diamond, Not Graphite
The most stable form of carbon at the low pressures used in a CVD chamber is actually graphite, not diamond. The success of the process hinges on one critical factor: preventing graphite from forming.
The Role of Hydrogen
This is where the high concentration of hydrogen gas becomes essential. While carbon atoms are settling on the substrate, hydrogen atoms are performing two crucial functions.
First, they bond to any carbon atoms that form weaker, graphite-like bonds. This process essentially "etches" away or cleans off the non-diamond carbon before it can disrupt the crystal structure.
Second, hydrogen stabilizes the diamond surface, preparing it to accept new carbon atoms into the correct diamond lattice. This selective process is what allows a high-quality diamond crystal to grow under conditions where it would not naturally form.
Understanding the Trade-offs and Key Parameters
The CVD process is a delicate balance of competing factors. Adjusting these parameters allows engineers to optimize the final product for different applications, from industrial coatings to flawless gemstones.
Temperature and Pressure
The substrate itself is heated, but to a much lower temperature (typically 900-1400°C) than the plasma. This temperature gradient is crucial for encouraging deposition on the seed. The chamber's low pressure allows the atoms to travel freely from the plasma to the substrate.
Purity vs. Growth Rate
Generally, growing diamond faster can lead to more defects or impurities in the crystal lattice. The highest-purity diamonds, often desired for advanced electronics or scientific applications, are typically grown very slowly to ensure each atom settles perfectly into place.
CVD vs. HPHT (High-Pressure, High-Temperature)
CVD should not be confused with the other primary method for creating diamonds, HPHT. HPHT mimics the natural geological process, using immense pressure and high temperatures to convert solid carbon (like graphite) into diamond. In contrast, CVD builds the diamond from a gas, atom by atom.
Making the Right Choice for Your Goal
The specific parameters of the CVD process are tuned based on the desired outcome. Understanding your primary goal is key to evaluating the technology.
- If your primary focus is producing large, high-purity single crystals for optics or electronics: CVD is the superior method, as it allows for unparalleled control over impurities and crystal structure.
- If your primary focus is creating durable industrial coatings on complex shapes: CVD is highly effective for depositing uniform, hard layers of polycrystalline diamond over large surface areas.
- If your primary focus is growing gem-quality diamonds for jewelry: Both CVD and HPHT are used, with CVD often favored for its ability to produce highly pure and colorless stones.
Ultimately, mastering the CVD process is about orchestrating a precise atomic ballet to build one of the world's most remarkable materials from the ground up.
Summary Table:
| Key CVD Process Step | Function | Key Parameter |
|---|---|---|
| Vacuum Chamber | Creates a controlled, contaminant-free environment | Pressure Control |
| Precursor Gas (e.g., Methane) | Provides the source of carbon atoms | Gas Composition & Flow Rate |
| Energy Source (e.g., Microwaves) | Creates plasma to break down gas molecules | Temperature & Power |
| Diamond Seed/Substrate | Acts as a crystalline template for growth | Seed Quality & Temperature |
| Hydrogen Gas | Etches non-diamond carbon and stabilizes growth | Hydrogen Concentration |
Ready to integrate precise CVD diamond technology into your laboratory or production line?
At KINTEK, we specialize in providing advanced lab equipment and consumables tailored to your research and manufacturing needs. Whether you are developing next-generation electronics, creating durable industrial coatings, or growing high-purity crystals, our expertise can help you optimize your CVD process for superior results.
Contact our experts today via our form to discuss how our solutions can bring unmatched purity and control to your diamond synthesis projects.
Visual Guide
Related Products
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- What are the primary advantages of the CVD method for growing diamonds? Engineering High-Purity Gems and Components
- How does Microwave Plasma Chemical Vapour Deposition (MPCVD) work? Your Guide to High-Purity Diamond Film Growth
- What is the frequency of MPCVD? A Guide to Choosing 2.45 GHz vs. 915 MHz for Your Application
- What is the difference between MPCVD and HFCVD? Choose the Right CVD Method for Your Application
- What is MP CVD? Unlock the Power of Microwave Plasma for High-Purity Diamond Synthesis



















