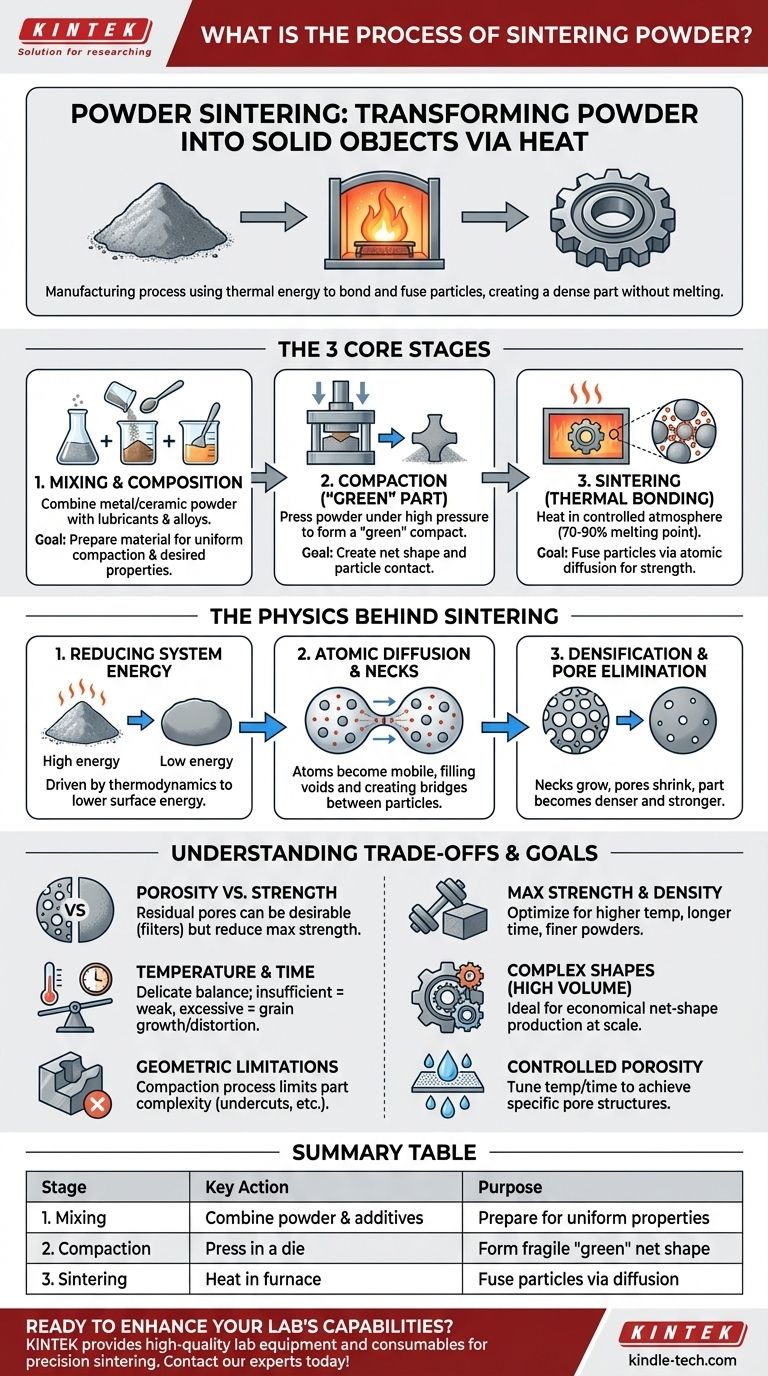
In essence, powder sintering is a manufacturing process that transforms a mass of metal or ceramic powder into a solid, coherent object using heat. The core stages involve preparing and mixing the powder, compressing it into a desired shape, and then heating this "green" part in a furnace to a temperature just below its melting point, causing the individual particles to bond and fuse together.
Sintering's fundamental goal is to create a dense, strong part from powder without ever melting the material. It achieves this by using thermal energy to drive atomic diffusion, which fuses particles together, reduces internal voids, and strengthens the final component.

The Three Core Stages of Powder Sintering
The process is remarkably consistent and can be broken down into three distinct phases, each critical to the final part's properties.
Stage 1: Material Composition and Mixing
Before any shaping can occur, the raw material must be prepared. This involves selecting a primary metal or ceramic powder, such as iron, copper, nickel, or alumina, based on the desired mechanical and physical properties of the end product.
Often, other materials are mixed in. Lubricants are added to improve the flow of the powder and reduce die wear during compaction, while specific alloying elements can be blended in to enhance strength, hardness, or corrosion resistance.
Stage 2: Compaction – Forming the "Green" Part
The prepared powder mix is then loaded into a die and compressed under significant pressure. This step forms the powder into a pre-sintered, fragile object known as a "green" compact or "green" part.
This part has the desired net shape but possesses only minimal strength, often just enough to be handled and transported to the furnace. The primary goal of compaction is to create particle-to-particle contact and establish the part's geometry.
Stage 3: Sintering – The Thermal Bonding Process
This is the heart of the process. The green part is placed in a furnace with a controlled atmosphere (to prevent oxidation) and heated to a high temperature, typically 70-90% of the material's absolute melting point.
The part is held at this temperature for a set period. During this time, the material does not melt. Instead, atoms migrate across the boundaries of the particles, a process called solid-state diffusion. This atomic movement creates "necks" or bridges between particles, which grow and cause the particles to fuse, eliminating the pores between them and densifying the object.
The Physics Behind Sintering: How Does It Work?
Understanding the "why" behind sintering reveals a process driven by fundamental thermodynamics and atomic-level mechanics.
The Driving Force: Reducing System Energy
A pile of loose powder has an incredibly high total surface area, which corresponds to a high state of surface energy. Like a ball rolling downhill, physical systems naturally seek their lowest possible energy state.
Sintering provides the thermal energy needed to activate the process of reducing this surface area. By fusing into a solid mass, the material drastically lowers its total surface energy, achieving a more stable state.
Atomic Diffusion and Neck Formation
At sintering temperatures, atoms at the contact points between particles become mobile. They begin to diffuse, moving from the bulk of one particle to fill the void at the "neck" between it and its neighbor.
This gradual transfer of material causes the necks to grow, pulling the particle centers closer together. The result is a progressive shrinking of the voids and an increase in the density of the component.
Densification and Pore Elimination
As the necks between particles grow and coalesce, the network of pores within the green part begins to shrink and become more isolated. The part becomes denser, stronger, and harder.
The extent of this densification is a function of temperature, time, and initial particle size. The process can be controlled to produce a fully dense part or one with a specific level of controlled porosity.
Understanding the Trade-offs
Sintering is a powerful technology, but it involves key considerations and limitations that must be managed.
Porosity vs. Strength
While the goal is often to eliminate porosity, some residual pores almost always remain. This means a sintered part may not achieve the full theoretical density or strength of a component made from wrought or cast metal. However, this porosity can be a desirable feature for applications like self-lubricating bearings or filters.
Temperature and Time Control
The sintering cycle is a delicate balance. Insufficient heat or time results in weak bonds and poor densification. Excessive heat or time can cause grain growth that degrades mechanical properties, or even lead to slumping and distortion if the material begins to melt. Precise control is non-negotiable.
Geometric Limitations
The initial compaction stage largely dictates the complexity of the part that can be produced. Features like undercuts or transverse holes are difficult or impossible to form with traditional die compaction, which primarily applies force in a single axis.
Making the Right Choice for Your Goal
Your application's primary driver will determine how you approach the sintering process.
- If your primary focus is maximum strength and density: You will need to optimize for higher sintering temperatures, longer hold times, and potentially use finer powders, which sinter more readily.
- If your primary focus is producing complex shapes in high volume: Your design must be compatible with the die compaction process, and sintering becomes the clear choice for economically creating that net shape at scale.
- If your primary focus is creating controlled porosity: Sintering is the ideal method, as you can precisely tune the temperature and time to stop the densification process and achieve a target pore structure for applications like filters or wicks.
Ultimately, powder sintering is a sophisticated method for converting powder into precise, functional components by manipulating energy and atomic motion.
Summary Table:
| Stage | Key Action | Purpose |
|---|---|---|
| 1. Mixing | Combine metal/ceramic powder with lubricants/alloys | Prepare material for uniform compaction and desired properties |
| 2. Compaction | Press powder in a die under high pressure | Form a fragile "green" part with the net shape |
| 3. Sintering | Heat green part in a controlled atmosphere furnace | Fuse particles via atomic diffusion to create a strong, dense object |
Ready to enhance your lab's capabilities with precision sintering?
KINTEK specializes in providing the high-quality lab equipment and consumables essential for successful powder sintering processes. Whether you are developing new materials or manufacturing complex components, our expertise and reliable products support every stage—from precise powder mixing to controlled thermal treatment.
Contact our experts today to discuss how we can help you achieve stronger, more consistent results and optimize your sintering workflow.
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- What is the development of thin film? From 17th Century Experiments to Atomic-Scale Engineering
- What are the impurities in pyrolysis oil? Unlocking the Complex Chemistry of Bio-Crude
- What is sintering of metallic powders? Fuse Metal Particles for High-Performance Components
- Is sintering the same as welding? Key Differences in Material Bonding and Fusion Explained
- Why is sintering needed? Create High-Performance Components Without Melting
- Is CBD Distillate the same as CBD oil? Understanding the Ingredient vs. the Final Product
- Is pyrolysis renewable? The answer lies in the feedstock you use.
- What 5 safety precautions should be taken when heating anything in the lab? Essential Rules for Lab Safety



















