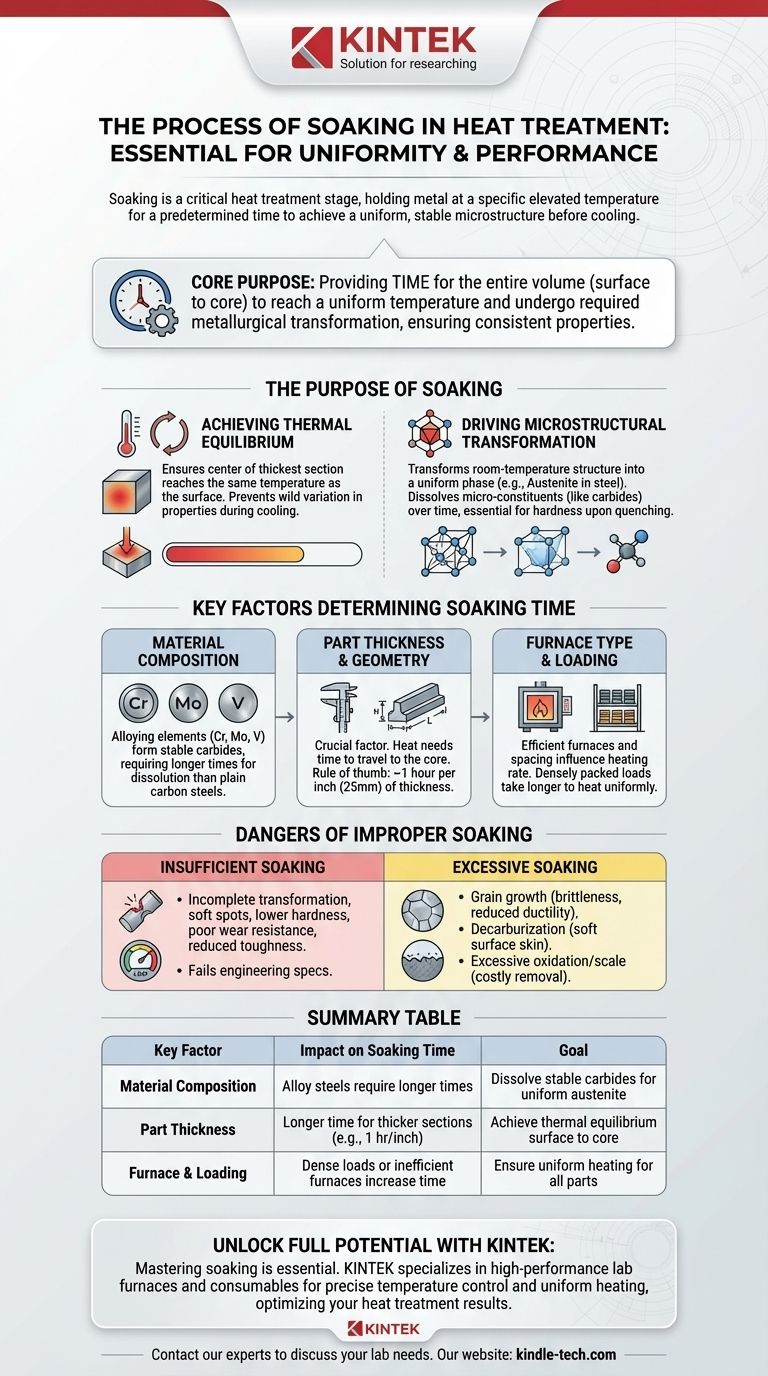
In metallurgy, soaking is the essential stage in a heat treatment cycle where a metal is held at a specific, elevated temperature for a predetermined length of time. This holding period is not passive; it is a critical step that allows the material's internal microstructure to achieve a uniform and stable state before it is cooled. The success of the entire heat treatment process, whether for hardening, softening, or stress relieving, often depends on getting this stage right.
The core purpose of soaking is to provide the necessary time for the entire volume of a part—from its surface to its core—to reach a uniform temperature and undergo the required metallurgical transformation, ensuring predictable and consistent properties throughout the material.

The Purpose of Soaking: Beyond Just Heating
Simply reaching a target temperature is not enough to change a material's properties effectively. The internal structure requires time to react and stabilize. Soaking ensures this transformation is complete and uniform.
Achieving Thermal Equilibrium
The surface of a metal part always heats faster than its core. The initial part of the soaking period allows the heat to penetrate fully, ensuring the center of the thickest section reaches the same temperature as the surface. Without this equalization, subsequent cooling would produce wildly different properties in different areas of the part.
Driving Microstructural Transformation
This is the most critical function of soaking. At elevated temperatures, the crystalline structure of metals changes. In steel, for example, the goal of a hardening process is to transform the room-temperature structure into a uniform structure called austenite.
This process involves dissolving carbon-rich micro-constituents (like carbides) into the iron matrix. This is analogous to dissolving sugar in water—it doesn't happen instantly. Soaking provides the necessary time for this dissolution to complete, creating a homogenous austenitic structure, which is the essential starting point for achieving high hardness upon quenching.
Key Factors That Determine Soaking Time
Calculating the correct soaking time is a balance of several factors. It is not a one-size-fits-all parameter and must be adjusted based on the material and the part itself.
Material Composition and Alloying Elements
Plain carbon steels transform relatively quickly. However, alloying elements like chromium, molybdenum, and vanadium form very stable carbides. These carbides are resistant to dissolving and require significantly longer soaking times or higher temperatures to form a uniform austenite.
Part Thickness and Geometry
The single most significant factor is the cross-sectional thickness of the part. Heat needs time to travel to the core. A common rule of thumb for steel is to soak for one hour for every inch (25 mm) of thickness, but this is only a starting point and is adjusted based on the other factors.
Furnace Type and Loading
The efficiency of the furnace and how parts are loaded also matters. A modern convection furnace may provide more uniform heating than an older radiant tube furnace. Likewise, densely packed parts will take longer to heat through than parts that are spaced apart, requiring a longer total furnace time to ensure every part is properly soaked.
Understanding the Trade-offs: The Dangers of Improper Soaking
Both insufficient and excessive soaking have severe negative consequences, making precision in this stage absolutely critical.
The Risk of Insufficient Soaking
If the soaking time is too short, the microstructural transformation will be incomplete. The core of the part may not reach the target temperature, or the necessary elements (like carbon) may not fully dissolve.
This results in a component with inconsistent properties. You may find soft spots, lower-than-expected hardness, poor wear resistance, and reduced toughness. The part will fail to meet its engineering specifications.
The Problem with Excessive Soaking
Soaking a part for too long is not only a waste of energy and money but can actively damage the material. Two primary risks are grain growth and adverse surface reactions.
Grain growth occurs when the microscopic crystals (grains) within the metal begin to merge and grow larger. Large grains make the steel more brittle and significantly reduce its toughness and ductility.
Furthermore, extended time at high temperatures can lead to decarburization, a process where carbon diffuses out of the surface of the steel. This creates a soft "skin" on the part, nullifying the hardening process where it's often needed most. It can also cause excessive surface oxidation, or scale, which may need to be removed in a costly secondary operation.
Making the Right Choice for Your Goal
The ideal soaking process is always tailored to the material and the desired outcome. There is no universal formula, only guiding principles.
- If your primary focus is maximum hardness in a simple carbon steel part: Ensure the core reaches temperature and allow just enough time for full austenitization, then proceed to quenching. A common guideline of 1 hour per inch of thickness is a reliable starting point.
- If your primary focus is toughening a complex alloy steel part: You must plan for longer soaking times to dissolve the stable alloy carbides, but carefully monitor time and temperature to prevent the brittleness caused by excessive grain growth.
- If your primary focus is stress relieving a welded assembly: The goal is different. Soaking occurs at a lower temperature and for a longer duration, aiming to relax internal stresses without causing a complete microstructural transformation.
Ultimately, mastering the soaking process is fundamental to controlling the final properties and unlocking the full performance potential of any heat-treated material.
Summary Table:
| Key Factor | Impact on Soaking Time | Goal |
|---|---|---|
| Material Composition | Alloy steels require longer times than plain carbon steels | Dissolve stable carbides for uniform austenite |
| Part Thickness | Longer time for thicker cross-sections (e.g., 1 hour per inch) | Achieve thermal equilibrium from surface to core |
| Furnace & Loading | Dense loads or inefficient furnaces increase time | Ensure uniform heating for all parts |
Unlock the Full Potential of Your Materials with KINTEK
Mastering the soaking process is essential for achieving the precise hardness, toughness, and durability your components require. Whether you're working with simple carbon steels or complex alloys, the right equipment and expertise make all the difference.
KINTEK specializes in high-performance lab furnaces and consumables designed for exacting heat treatment processes. Our solutions ensure precise temperature control and uniform heating, helping you avoid the pitfalls of improper soaking—like soft spots, brittleness, or decarburization.
Let us help you optimize your heat treatment cycles for consistent, high-quality results. Contact our experts today to discuss your specific laboratory needs and discover how KINTEK can enhance your metallurgical outcomes.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are zeolites advantages and disadvantages? Maximize Molecular Selectivity and Efficiency
- What is the most efficient way to remove excess low boiling point solvent from a high boiling point material? Use Rotary Evaporation for Fast, Safe Removal
- What are the two types of casting machines? Hot-Chamber vs. Cold-Chamber Die Casting
- What is the pressure of sputter coating? The Key to Optimizing Your Thin Film Deposition
- What are the advantages of using oil-free diaphragm vacuum pumps? Achieve Clean, Low-Maintenance Vacuum
- What are the applications of compressors? Powering Industries from Manufacturing to HVAC
- What are the effects of temperature in metal working process? Master Strength, Ductility, and Microstructure
- Can graphite withstand heat? Unlocking its extreme 3,600°C potential in inert environments



















