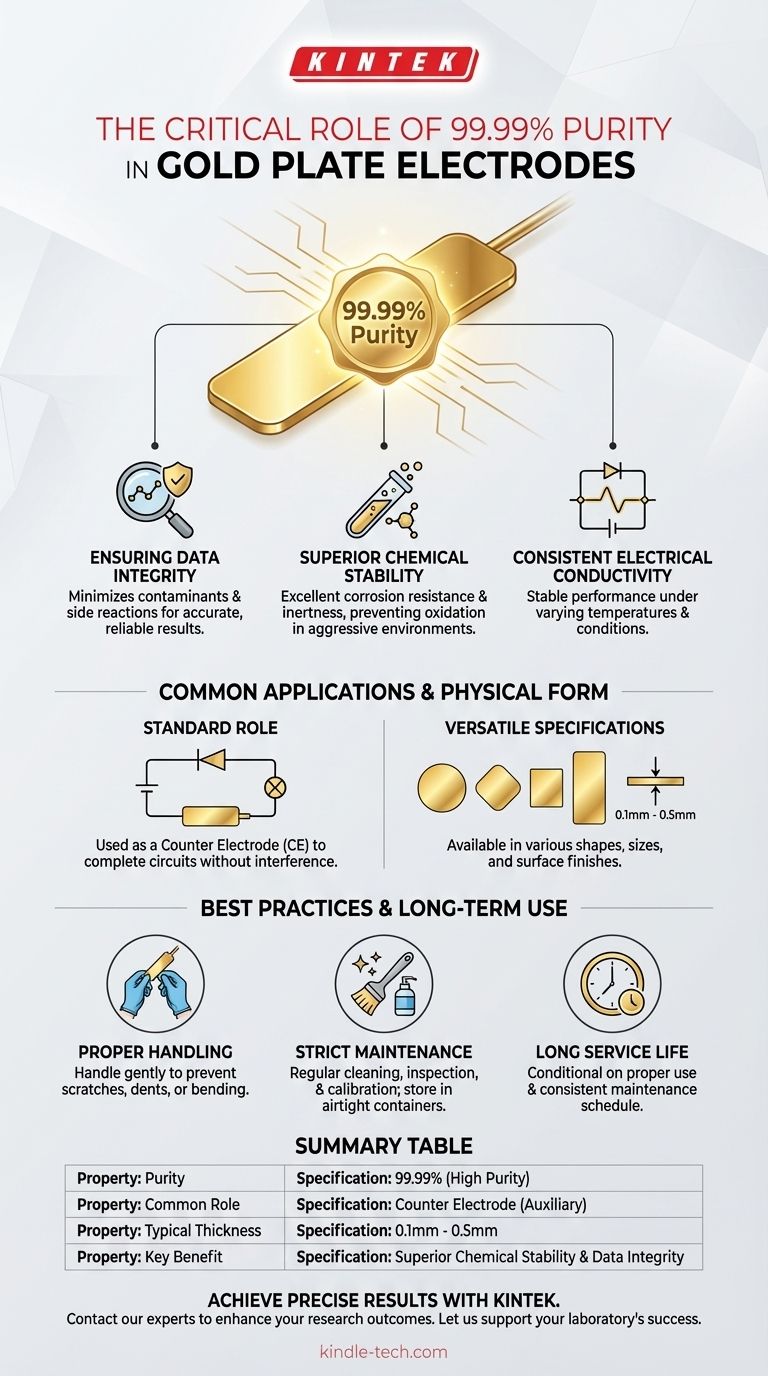
The purity of a standard gold plate electrode is 99.99%. This high level of purity is not merely a specification; it is the primary factor that ensures the accuracy, reliability, and reproducibility of experimental data in sensitive electrochemical applications.
The 99.99% purity of a gold plate electrode is the foundation of its value. This characteristic ensures the electrode remains chemically inert and electrically stable, preventing it from interfering with the very reactions it is meant to measure.

The Core Attributes of a 99.99% Gold Electrode
The purity figure directly translates into tangible performance characteristics that are critical for scientific research. These attributes ensure the electrode acts as a stable, predictable tool rather than an unwanted variable.
Ensuring Data Integrity
High purity minimizes the presence of contaminants or alloyed metals that could trigger unintended side reactions. This chemical cleanliness is essential for obtaining accurate and reliable results.
When the electrode material is pure, you can be confident that the electrochemical data reflects the reaction you are studying, not interference from impurities.
Superior Chemical Stability
Gold's inherent nobility is amplified at 99.99% purity. The electrode possesses excellent corrosion resistance and is not easily oxidized.
This stability means it maintains its structural and chemical integrity even in aggressive electrochemical environments, ensuring consistent performance over long-term use.
Consistent Electrical Conductivity
A pure gold plate electrode exhibits highly stable electrical conductivity. Its performance shows minimal variation under changing temperatures or other environmental conditions.
This predictability is crucial for experiments that rely on precise and consistent current flow.
Common Applications and Physical Form
Understanding the typical use case and physical options for a gold plate electrode provides context for why its material properties are so important.
A Standard Role as a Counter Electrode
Due to its inertness and conductivity, the gold plate electrode is commonly used as a Counter Electrode (CE), also known as an auxiliary electrode.
In this role, its job is to complete the electrical circuit without reacting or interfering with the primary electrochemical process occurring at the working electrode.
Versatile Physical Specifications
These electrodes are not one-size-fits-all. They are typically offered as thin sheets with a thickness from 0.1mm to 0.5mm.
They come in various shapes, such as circular, square, or rectangular, with customizable sizes and surface finishes (e.g., polished or roughened) to suit specific experimental setups.
Best Practices and Long-Term Use
While the material itself is robust, a high-purity gold electrode is a precision instrument. Its longevity and performance are directly tied to proper handling and care.
The Importance of Proper Handling
The electrodes are delicate and must be handled gently to prevent scratches, dents, or bending, which could alter their electrochemical properties.
A Strict Maintenance Regimen
Performance depends on a clean surface. Regular maintenance should include cleaning, inspection, and calibration based on the frequency of use.
For storage, the electrode must be thoroughly dried and kept in a dedicated, airtight container to protect it from atmospheric contaminants.
Long Service Life is Conditional
With proper use and a consistent maintenance schedule, the gold plate electrode can have a very long service life. Neglecting this care is the fastest way to compromise its performance.
Making the Right Choice for Your Goal
Your primary objective dictates which aspect of the gold electrode is most critical to your work.
- If your primary focus is experimental accuracy: The 99.99% purity is your guarantee against contamination and side reactions, ensuring clean, reliable data.
- If your primary focus is long-term stability: Its excellent corrosion resistance and chemical inertness mean it will perform consistently over many experiments, provided it is maintained.
- If your primary focus is application versatility: The availability of various sizes, shapes, and surface finishes allows you to select the ideal physical form for your specific electrochemical cell.
Understanding these properties allows you to leverage the gold plate electrode as a reliable tool for achieving precise and reproducible scientific outcomes.
Summary Table:
| Property | Specification |
|---|---|
| Purity | 99.99% (High Purity) |
| Common Role | Counter Electrode (Auxiliary) |
| Typical Thickness | 0.1mm - 0.5mm |
| Key Benefit | Superior Chemical Stability & Data Integrity |
Achieve precise and reliable results with high-purity electrodes from KINTEK.
Your research demands the highest standards. KINTEK specializes in premium lab equipment and consumables, providing electrodes and materials that ensure data integrity and long-term stability for your sensitive electrochemical applications.
Let us support your laboratory's success. Contact our experts today to discuss your specific needs and discover how our products can enhance your research outcomes.
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What is the operating principle of a gold disc electrode in an electrochemical system? Unlock Precision with a Stable Interface
- How should a gold disc electrode be handled during an experiment? Ensure Accurate Electrochemical Measurements
- What is the material and purity of a gold disc electrode? Ensuring Precision in Electrochemical Analysis
- What are the key precautions for a gold disc electrode? Ensure Accurate Results & Long Lifespan
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data



















