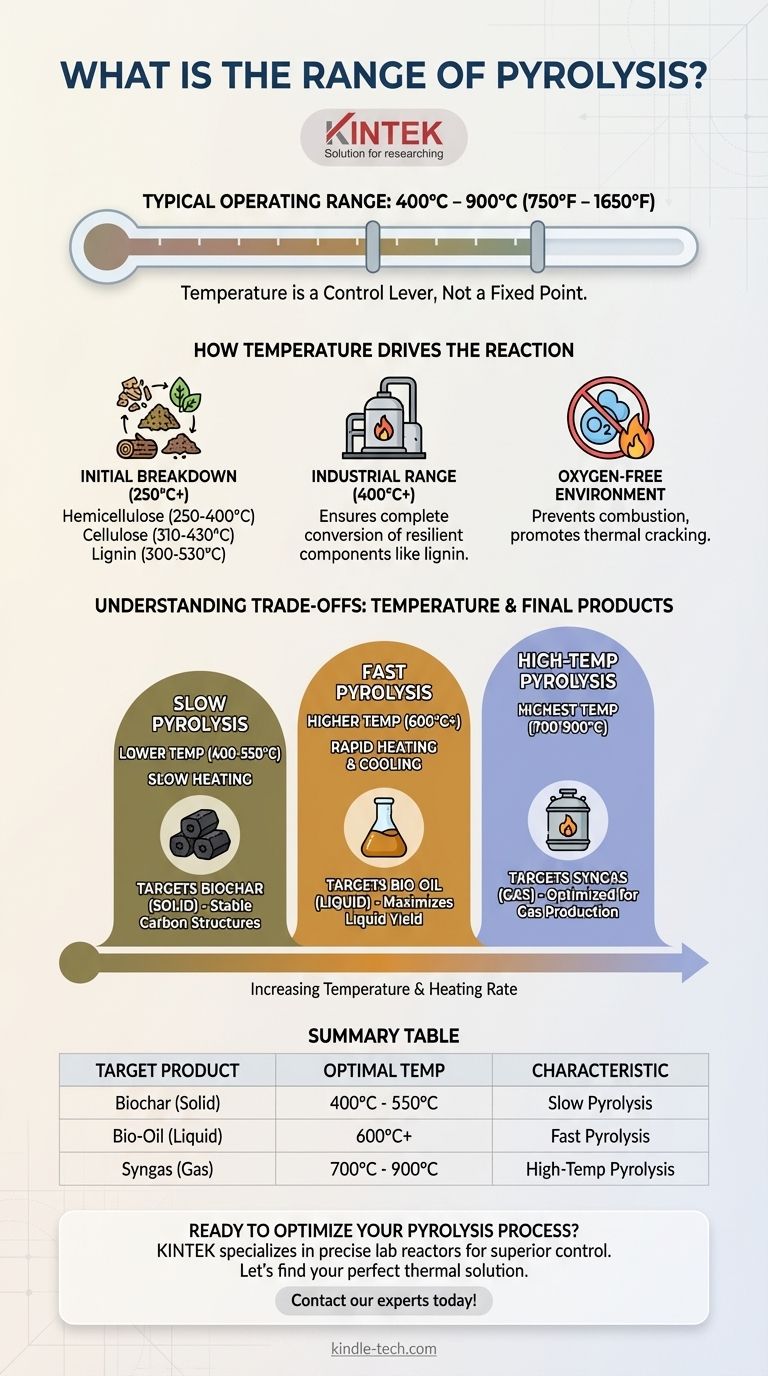
The typical operating range for pyrolysis is between 400°C and 900°C (750°F to 1650°F). This temperature is not a single setpoint but a crucial variable that depends heavily on the specific material being processed and the desired end products. The initial thermal decomposition of organic matter, however, begins at much lower temperatures.
Pyrolysis temperature is best understood not as a fixed number, but as a control lever. Adjusting the heat within its effective range directly determines whether the process yields more solid biochar, liquid bio-oil, or combustible gases from the raw biomass.

How Temperature Drives the Pyrolysis Reaction
Pyrolysis is fundamentally a process of thermal decomposition. To understand the wide temperature range, we must first look at what happens to the core components of organic material, like biomass, as heat is applied in an oxygen-free environment.
The Initial Breakdown of Biomass Components
Biomass is primarily composed of three main structures: hemicellulose, cellulose, and lignin. Each of these breaks down at a different temperature threshold.
- Hemicellulose is the least stable and begins to decompose first, typically between 250°C and 400°C.
- Cellulose, the main structural component of plant cells, decomposes in a narrower and slightly higher range of 310°C to 430°C.
- Lignin is the most complex and robust component, breaking down slowly across a very broad range from 300°C up to 530°C.
Why the Operating Range is Higher
While the initial breakdown starts as low as 250°C, industrial pyrolysis processes operate at higher temperatures (400°C+) to ensure a complete and efficient conversion.
Operating in this higher range ensures that even the most resilient components, like lignin, are fully broken down. This maximizes the transformation of the raw material into more valuable products.
The Role of an Oxygen-Free Environment
Crucially, this heating occurs in the absence of oxygen. This prevents combustion (burning). Instead of turning into ash and smoke, the organic material thermally cracks apart into smaller molecules, which can be collected as solids, liquids, and gases. The ultimate goal is to remove water and oxygen, preserving as much useful carbon as possible.
Understanding the Trade-offs: Temperature and Final Products
The specific temperature chosen within the 400°C to 900°C range is a deliberate choice that dictates the output of the system. This is the most critical trade-off in process design.
Targeting Lower Temperatures (Slow Pyrolysis)
When pyrolysis is conducted at the lower end of the range (e.g., 400-550°C) over a longer period, the process favors the production of biochar. The slower heating rates allow the carbon to arrange into stable, solid structures.
Targeting Higher Temperatures (Fast Pyrolysis)
Conversely, very high temperatures (e.g., 600-700°C and above) and rapid heating rates cause the biomass to vaporize almost instantly. These vapors, when rapidly cooled and condensed, form a liquid known as bio-oil. This process is optimized to maximize liquid yield.
Making the Right Choice for Your Goal
Selecting the correct temperature is essential for achieving your desired outcome efficiently.
- If your primary focus is maximizing solid biochar: You should operate at the lower end of the pyrolysis range (approx. 400-550°C) with slower heating rates.
- If your primary focus is producing liquid bio-oil: You require higher temperatures (often above 600°C) and a reactor designed for extremely fast heat transfer.
Ultimately, mastering the pyrolysis process begins with understanding that temperature is the primary tool for directing the chemical outcome.
Summary Table:
| Target Product | Optimal Temperature Range | Key Process Characteristic |
|---|---|---|
| Biochar (Solid) | 400°C - 550°C | Slow Pyrolysis |
| Bio-Oil (Liquid) | 600°C+ | Fast Pyrolysis |
| Syngas (Gas) | 700°C - 900°C | High-Temperature Pyrolysis |
Ready to optimize your pyrolysis process?
Selecting the right temperature is critical for maximizing your yield of biochar, bio-oil, or syngas. KINTEK specializes in high-quality lab reactors and furnaces that deliver the precise, consistent heating required for successful pyrolysis.
Our equipment helps researchers and engineers like you achieve superior control and efficiency. Let's discuss your project requirements and find the perfect thermal solution for your lab.
Contact our experts today for a consultation!
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory High Pressure Vacuum Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- How do high-temperature reaction furnaces control in-situ MMCs? Master Material Precision and Structural Integrity
- What are the factors affecting the yield of bio-oil from the pyrolysis of coconut shell? Control 4 Key Parameters
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- How is a high-temperature calcination furnace utilized in BZY20 Sol-gel? Achieve Pure Cubic Perovskite Phases
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies



















