In any heat-treating process, water vapor is a highly reactive chemical agent, not an inert bystander. Its primary role is to react with the surface of steel and other materials inside the furnace, typically causing oxidation. This reactivity is significant even at extremely low concentrations and pressures.
The presence of water vapor in a furnace is never neutral. It is a powerful oxidizing or decarburizing agent that must be meticulously controlled to prevent unwanted surface reactions and ensure the quality, integrity, and desired properties of the final product.

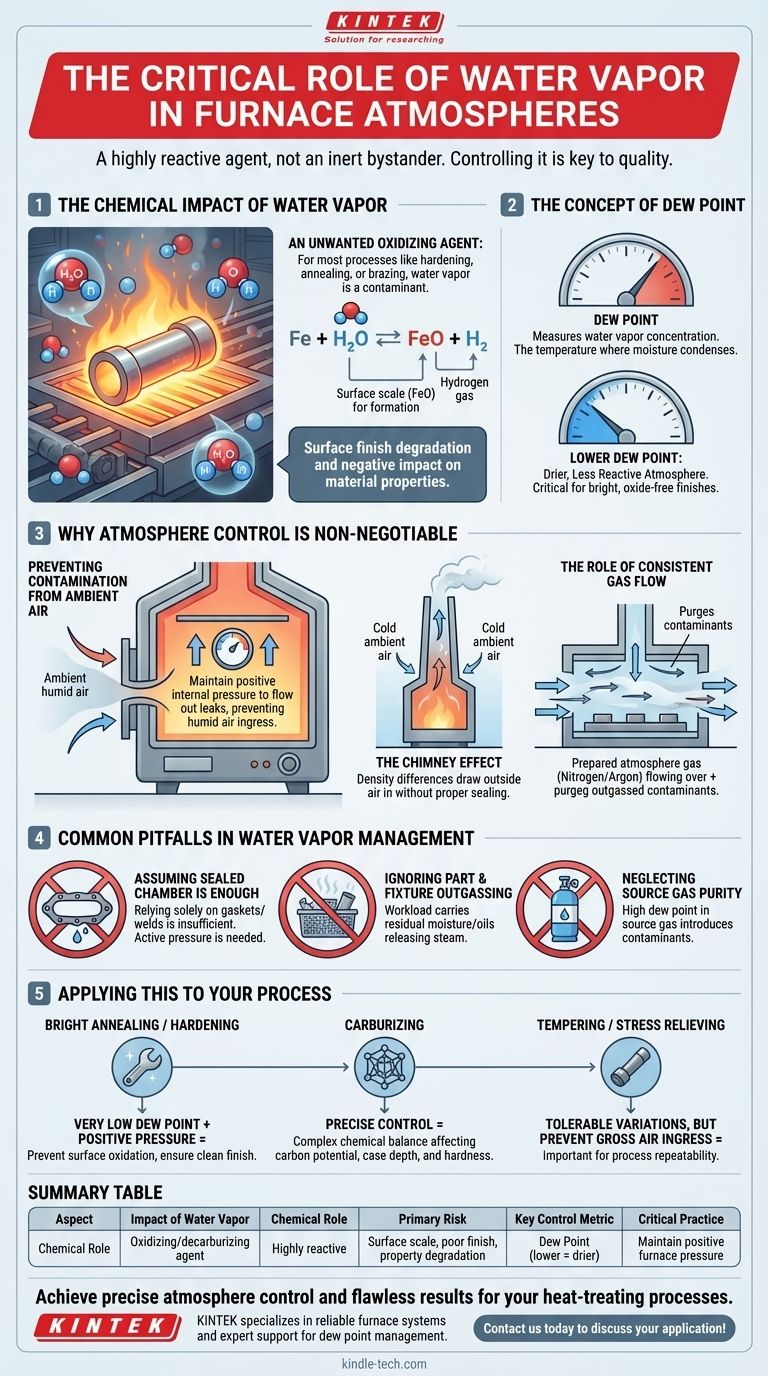
The Chemical Impact of Water Vapor
Water vapor directly influences the surface chemistry of the parts being treated. Understanding its role is fundamental to achieving the desired metallurgical outcome.
An Unwanted Oxidizing Agent
For most processes like hardening, annealing, or brazing, water vapor is a contaminant. It readily reacts with iron (Fe) at high temperatures to form iron oxides (scale) and hydrogen gas.
This reaction, Fe + H₂O ⇌ FeO + H₂, degrades the surface finish and can negatively impact the material's properties.
The Concept of Dew Point
The concentration of water vapor in a furnace atmosphere is measured by its dew point—the temperature at which the moisture would condense.
A lower dew point signifies a drier, less reactive atmosphere. For processes requiring a bright, oxide-free finish, maintaining an extremely low dew point is critical.
Why Atmosphere Control is Non-Negotiable
Because water vapor is so reactive, preventing its unplanned entry into the furnace is a primary goal of atmosphere control systems. This involves managing both pressure and gas flow.
Preventing Contamination from Ambient Air
The most common source of water vapor contamination is the outside air.
Furnaces designed for controlled atmospheres must maintain a slight positive internal pressure. This ensures that if any small leaks exist, the controlled atmosphere gas flows out, rather than humid ambient air flowing in.
The 'Chimney Effect'
A lack of proper sealing and pressure can lead to the 'chimney effect'.
Density differences between the hot furnace gas and the cooler ambient air create buoyancy. This can draw outside air into the furnace, introducing a constant and uncontrolled stream of oxygen and water vapor.
The Role of Consistent Gas Flow
Controlling the flow of the prepared atmosphere gas (like nitrogen, argon, or endothermic gas) is another critical factor.
A steady, engineered flow pattern helps purge any contaminants that enter the chamber or are released from the parts themselves (outgassing). This ensures a consistent chemical environment at the part's surface.
Common Pitfalls in Water Vapor Management
Effective atmosphere control requires vigilance against common points of failure that can introduce unwanted water vapor.
Assuming a Sealed Chamber is Enough
No furnace is perfectly sealed. Relying solely on gaskets and welds is insufficient. Active measures like maintaining positive pressure are the only reliable defense against leaks.
Ignoring Part and Fixture Outgassing
The workload itself, along with any fixtures or baskets, can carry residual moisture or oils that release water vapor when heated. A sufficient gas flow and purge cycle at the beginning of a run is necessary to remove these contaminants.
Neglecting Source Gas Purity
The gas used to create the atmosphere must be sufficiently dry. If the source gas has a high dew point, you are introducing the contaminant you are trying to eliminate.
Applying This to Your Process
The required level of water vapor control is dictated entirely by your metallurgical goal.
- If your primary focus is bright annealing or hardening: You must maintain a very low dew point and positive furnace pressure to prevent any surface oxidation and ensure a clean finish.
- If your primary focus is carburizing: Water vapor is part of a complex chemical balance that affects carbon potential, and its level must be precisely measured and controlled to achieve the correct case depth and hardness.
- If your primary focus is tempering or stress relieving (non-critical surface): While still important, slight variations in water vapor might be tolerable, but preventing gross air ingress is still mandatory for process repeatability.
Ultimately, mastering your furnace atmosphere begins with understanding and controlling its most reactive component: water vapor.
Summary Table:
| Aspect | Impact of Water Vapor |
|---|---|
| Chemical Role | Oxidizing/decarburizing agent |
| Primary Risk | Surface scale, poor finish, property degradation |
| Key Control Metric | Dew Point (lower = drier atmosphere) |
| Critical Practice | Maintain positive furnace pressure |
Achieve precise atmosphere control and flawless results for your heat-treating processes. KINTEK specializes in lab equipment and consumables, providing the reliable furnace systems and expert support your laboratory needs to master dew point management and prevent contamination. Contact us today to discuss your application and ensure your process integrity!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the role of using a high-temperature atmosphere furnace for the pre-carbonization of viscose-based fibers? Achieve High-Performance Carbon-Carbon Composites
- What is inert atmosphere used for? Prevent Oxidation and Ensure Process Safety
- What is an example of a reducing atmosphere? Learn How It Transforms Materials in Industry
- Why is a high-pressure nitrogen environment of 1 to 3 MPa required for Si2N2O synthesis? Optimize Ceramic Phase Purity
- What is a hydrogen oven? The Future of Clean, High-Temperature Cooking
- How does a Tube Atmosphere Furnace ensure noble metal activity? Key Steps for Preparing Pt/Al2O3 Catalysts
- What is an exothermic atmosphere? A Guide to Cost-Effective, Self-Sustaining Heat Treatment Gases
- What is the function of a high vacuum atmosphere furnace in validating hydrogen diffusion models? Ensure Pure Data.



















