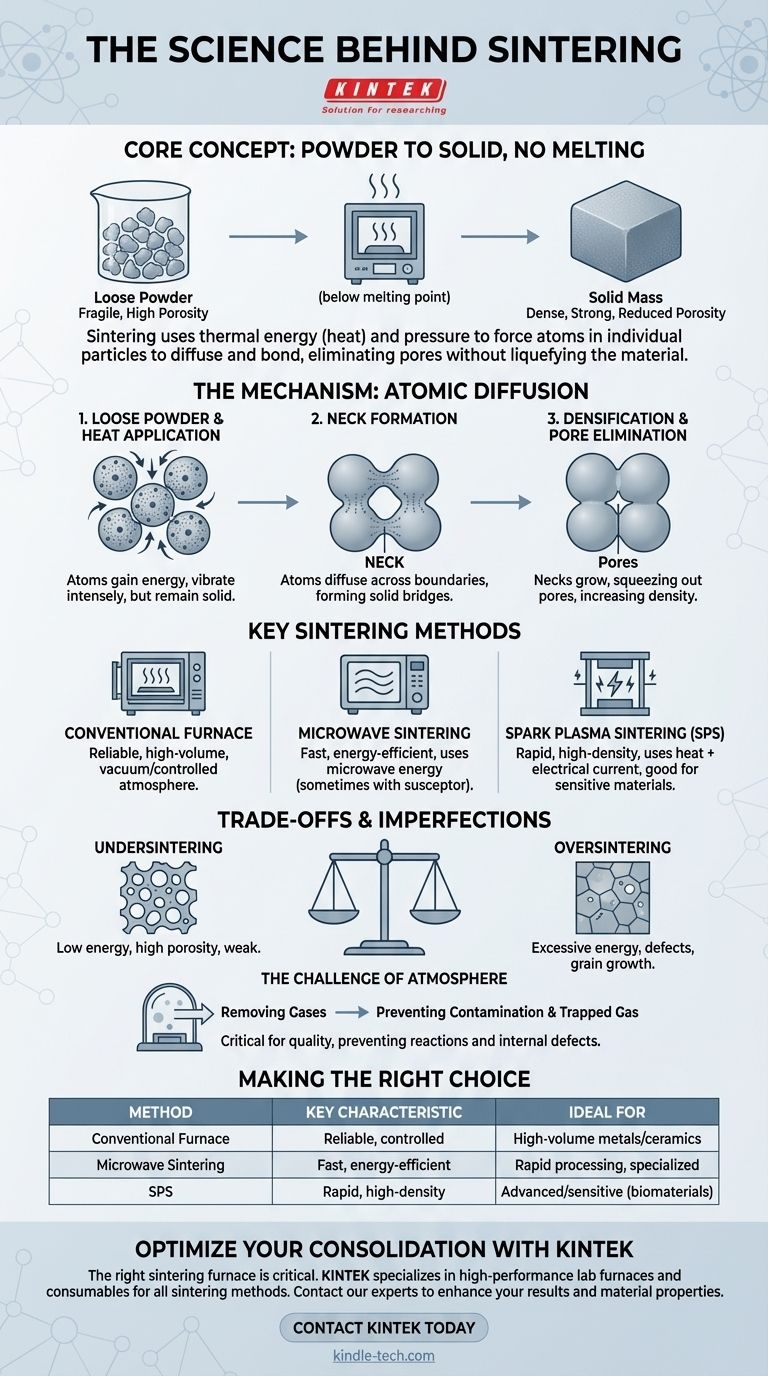
At its core, sintering is a thermal process that transforms a powder into a solid mass without melting it. It uses heat and pressure to force atoms in individual particles to diffuse and bond across their boundaries. This atomic migration effectively fuses the particles, reduces the empty space between them, and creates a dense, solid object from a loose starting material.
Sintering is not about melting; it's about atomic movement. By applying energy—usually heat—below a material's melting point, you enable atoms to migrate across particle boundaries, eliminating pores and creating a single, solid piece from a collection of smaller ones.

The Fundamental Mechanism: Atomic Diffusion
Sintering may seem complex, but it hinges on a few core scientific principles that govern how solid materials behave when heated. The entire process is a carefully controlled journey from a loose powder to a dense, solid structure.
From Loose Powder to a Solid Structure
The process begins with a mass of loose particles, often compacted into a desired shape. At this stage, the object is fragile, held together only by friction, with significant empty space, or porosity, between the individual grains.
The Role of Energy (Heat)
When heat is applied, the atoms within each particle gain energy and begin to vibrate more intensely. Crucially, the temperature remains below the material's melting point. This energy doesn't liquefy the material but makes the atoms mobile enough to move.
Neck Formation: The First Bond
At the points where particles touch, this increased atomic mobility allows atoms to migrate or diffuse across the boundary from one particle to another. This creates a small bridge of solid material, known as a neck. The formation of these necks is the first step in fusing the particles together.
Eliminating Porosity for Higher Density
As the sintering process continues, these necks grow wider. This growth pulls the centers of the particles closer together, systematically squeezing out the pores and empty channels between them. The result is a significant increase in the material's overall relative density and strength.
Key Methods of Sintering
While the underlying principle of atomic diffusion is universal, several methods exist to apply the necessary energy and control the environment.
Conventional Furnace Sintering
This is the most common method, where the material is heated in a high-temperature furnace, such as a mesh belt or walking-beam furnace. Often, this is performed in a vacuum to remove atmospheric gases that could react with the material or become trapped, creating imperfections.
Microwave Sintering
This modern technique uses microwave energy to generate heat directly within the material. For materials that do not absorb microwaves efficiently (like certain ceramics), a susceptor material is used to absorb the energy and convert it to heat. This method can be significantly faster than conventional sintering.
Spark Plasma Sintering (SPS)
SPS is an advanced, rapid consolidation technique often used for high-performance or sensitive materials like biomaterials. It uses a combination of heat and electrical current to achieve high densities in a very short amount of time, which helps prevent unwanted structural changes in the material.
Understanding the Trade-offs and Imperfections
Achieving a perfect, fully dense final product is the goal of sintering, but the process must be precisely controlled to avoid common pitfalls.
Undersintering vs. Oversintering
Finding the right balance of temperature and time is critical. Undersintering occurs when there isn't enough energy for full diffusion, leaving the final part porous, weak, and with poor mechanical properties. Oversintering, on the other hand, can cause defects like blistering, sweating, or excessive grain growth that can also weaken the material.
The Challenge of Porosity
While the goal is to eliminate pores, achieving 100% density is often difficult or impractical. The key is to control the final porosity—the amount, size, and distribution of any remaining voids—to ensure the final product meets its required specifications for strength, permeability, or other properties.
The Need for Atmosphere Control
Performing sintering in a vacuum is not just for efficiency; it's for quality. Removing atmospheric gases prevents them from reacting with the hot material, which could cause contamination. It also ensures that gas doesn't become trapped in the closing pores, which would inhibit the densification process and create internal defects.
Making the Right Choice for Your Goal
The best sintering method depends entirely on the material, the desired properties of the final part, and production constraints like speed and cost.
- If your primary focus is high-volume, established processes: Conventional furnace sintering in a controlled atmosphere provides reliable and well-understood results for materials like metals and ceramics.
- If your primary focus is speed and energy efficiency: Microwave sintering can dramatically reduce processing times, though it may require specialized equipment and material considerations.
- If your primary focus is advanced or sensitive materials: Specialized methods like Spark Plasma Sintering or high-vacuum sintering are used to achieve high density quickly while minimizing structural changes.
Ultimately, mastering sintering is about precisely controlling energy and environment to manipulate matter at the atomic level.
Summary Table:
| Sintering Method | Key Characteristic | Ideal For |
|---|---|---|
| Conventional Furnace | Reliable, controlled atmosphere | High-volume production of metals/ceramics |
| Microwave Sintering | Fast, energy-efficient | Rapid processing, specialized materials |
| Spark Plasma Sintering (SPS) | Rapid, high-density results | Advanced/sensitive materials like biomaterials |
Ready to achieve precise material consolidation in your lab? The right sintering furnace is critical for controlling atomic diffusion and eliminating porosity. KINTEK specializes in high-performance lab furnaces and consumables for sintering metals, ceramics, and advanced materials. Our experts can help you select the ideal equipment for your specific process, whether you need a conventional, microwave, or SPS solution. Contact our team today to discuss how we can enhance your sintering results and material properties.
Visual Guide
Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
People Also Ask
- What are the key functions of a vacuum hot press sintering furnace? Produce High-Density UN Ceramic Pellets
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context
- What are the primary advantages of using a vacuum hot pressing sintering furnace? Maximize Density in B4C-CeB6 Ceramics
- How does a vacuum environment system contribute to the hot pressing sintering of B4C-CeB6? Unlock Peak Ceramic Density
- What are the advantages of vacuum sintering? Achieve Superior Purity, Strength, and Performance



















