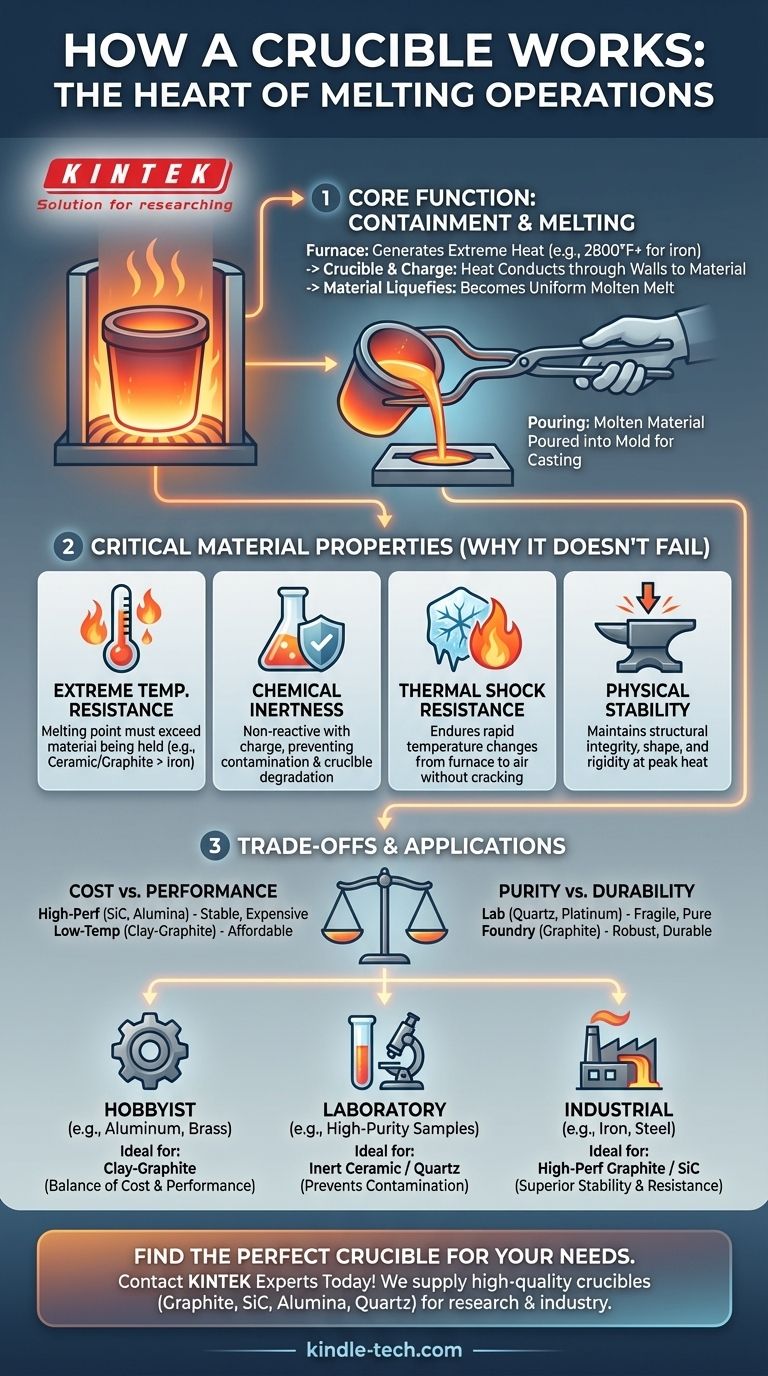
At its core, a crucible works by acting as a highly resilient container designed to hold materials while they are heated to their melting point within a furnace. The furnace generates extreme heat, which is transferred through the crucible's walls to the material inside, causing it to liquefy. Once molten, the material can be poured into a mold to create a new object.
The crucible is more than just a pot; it is a critical piece of technology whose success depends entirely on its material properties. Its primary function is to withstand extreme conditions—heat, chemical reactions, and physical stress—to contain a melt without failing or contaminating it.

The Core Function: Containment and Melting
A crucible is a passive component in an active process. It does not generate heat itself but is essential for managing the heat and the material being processed.
The Role of the Furnace
The crucible is placed inside a furnace, which provides the energy required for melting. The furnace can be powered by fuel (like propane or gas) or electricity (induction or resistance heating).
The Mechanism of Heat Transfer
The furnace heats the crucible, which then conducts that thermal energy into the material it holds, known as the charge. The temperature is carefully controlled and maintained until the entire charge becomes a uniform liquid.
The Final Step: Pouring
Once the material is fully molten, the crucible is carefully removed from the furnace using specialized tongs. The molten liquid is then poured into a pre-made mold, where it cools and solidifies into its final shape.
Why Material Selection is Critical
The true "working" of a crucible lies in its ability to withstand the harsh environment of a melt. Its material composition is therefore the most important factor.
Extreme Temperature Resistance
This is the most fundamental requirement. A crucible’s melting point must be significantly higher than the melting point of the material it is intended to hold. A crucible for melting iron (melts at ~2800°F / 1538°C) must be made of a material like ceramic or graphite that can endure much higher temperatures.
Chemical Inertness
Molten metals and glass are often highly reactive. A crucible must be chemically inert, meaning it will not react with the substance it holds. This prevents two critical problems: contamination of the melt and degradation of the crucible itself.
Thermal Shock Resistance
The crucible must endure rapid and extreme temperature changes without cracking. This property, known as thermal shock resistance, is vital as the crucible moves from a blazing-hot furnace into the much cooler ambient air during pouring.
Physical Stability
At peak temperatures, some materials can soften or lose their structural integrity. A crucible must remain physically stable and rigid, ensuring it doesn't sag, deform, or break while holding a heavy load of molten liquid.
Understanding the Trade-offs
Selecting a crucible involves balancing competing factors. There is no single "best" material for all applications.
Cost vs. Performance
High-performance crucibles made from materials like silicon carbide or high-purity alumina offer excellent stability and long life but are expensive. For low-temperature metals like aluminum or zinc, a more affordable clay-graphite or even a properly prepared steel crucible may be sufficient.
Purity vs. Durability
In a laboratory or semiconductor setting, purity is paramount, and a quartz or platinum crucible might be used despite being fragile. In a foundry, durability and the ability to withstand physical abuse for hundreds of cycles might be more important, even if it introduces microscopic impurities.
Making the Right Choice for Your Application
Your goal dictates the ideal crucible material.
- If your primary focus is hobbyist metal casting (e.g., aluminum, brass): A durable and cost-effective clay-graphite crucible offers the best balance of performance and affordability.
- If your primary focus is high-purity laboratory work: An inert ceramic like alumina or a specialized material like quartz is necessary to prevent any contamination of your sample.
- If your primary focus is industrial or high-temperature melts (e.g., iron, steel): A high-performance graphite or silicon carbide crucible is required for its superior thermal stability and resistance to chemical attack.
Ultimately, the crucible is the silent, indispensable heart of any high-temperature casting or melting operation.
Summary Table:
| Crucible Property | Why It Matters |
|---|---|
| Temperature Resistance | Must withstand temperatures far higher than the material being melted. |
| Chemical Inertness | Prevents contamination of the melt and degradation of the crucible. |
| Thermal Shock Resistance | Allows the crucible to survive rapid temperature changes without cracking. |
| Physical Stability | Maintains shape and integrity under the extreme heat and weight of the molten material. |
Ready to find the perfect crucible for your application?
Whether you're in a research lab requiring ultra-high purity or an industrial foundry needing robust, high-temperature performance, KINTEK has the expertise and product range to meet your needs. We specialize in supplying high-quality lab equipment and consumables, including crucibles made from materials like graphite, silicon carbide, alumina, and quartz.
Contact our experts today to discuss your specific requirements and let us help you select the crucible that ensures safety, efficiency, and purity in your melting processes.
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- Why are high-purity alumina crucibles used for LATP? Preserve Purity and Conductivity in Sintering
- What is the function of alumina crucibles in Na3V2(PO4)2F3 synthesis? Ensure Purity in NVPF Production
- How does the use of corrosion-resistant ceramic crucibles ensure the chemical purity of materials? | KINTEK
- What role do high-purity alumina crucibles play in high-temperature steam oxidation? Ensure Data Integrity up to 1350°C
- What are the functional advantages of using high-purity alumina crucibles? Achieve Precise Oxidation Data



















