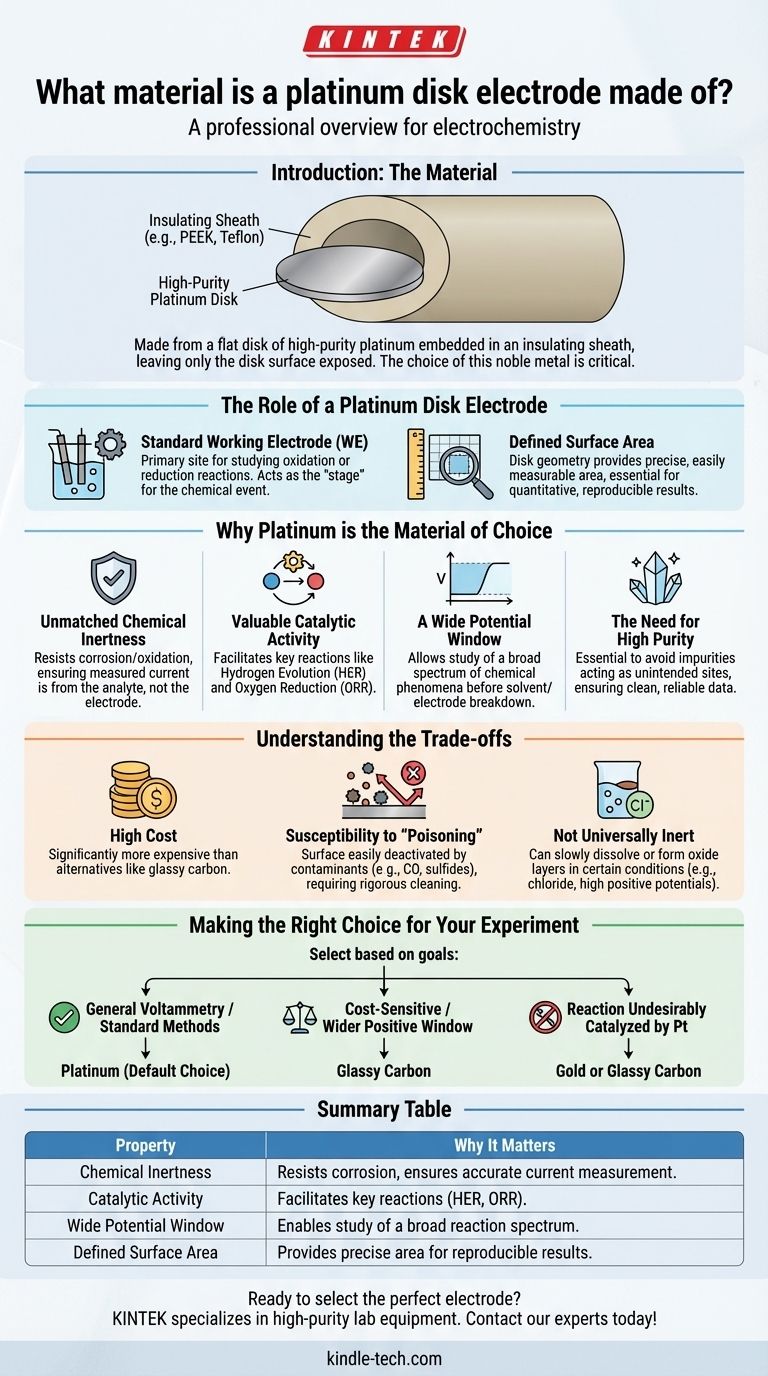
As its name implies, a platinum disk electrode is made from a small, flat disk of high-purity platinum. This metal is embedded in an insulating sheath, typically a polymer like PEEK or Teflon, leaving only the flat disk surface exposed to the solution.
While the material is simply platinum, the critical insight is why this specific noble metal is chosen. Platinum offers a unique and often ideal balance of chemical inertness and catalytic activity, making it a benchmark standard for a vast range of electrochemical experiments.

The Role of a Platinum Disk Electrode
To understand why platinum is used, we must first understand the electrode's function. It is not merely a piece of metal; it is a precision instrument for observing chemical reactions.
A Standard Working Electrode (WE)
In a typical three-electrode setup, the working electrode is the primary site of interest. It is where the specific oxidation or reduction reaction you are studying takes place.
The platinum disk electrode is one of the most common and well-characterized working electrodes used in electrochemistry, acting as the "stage" for the chemical event.
The Importance of a Defined Surface Area
The "disk" geometry is intentional and critical. It provides a well-defined and easily measurable surface area.
Because the rate of an electrochemical reaction is proportional to the current density (current per unit area), knowing the exact area is essential for quantitative, reproducible results.
Why Platinum is the Material of Choice
While many metals can conduct electricity, platinum possesses a unique combination of properties that make it exceptionally well-suited for its role as a working electrode.
Unmatched Chemical Inertness
Platinum is a noble metal, meaning it resists corrosion and oxidation in most environments. Over a wide range of applied voltages (potentials), it remains stable and does not interfere with the reaction being studied.
This ensures that the measured current comes from your analyte, not from the electrode itself dissolving or reacting.
Valuable Catalytic Activity
Unlike a completely passive material, platinum exhibits significant catalytic activity for many important reactions. It is particularly effective at facilitating the hydrogen evolution reaction (HER) and oxygen reduction reaction (ORR).
This makes it the ideal choice when the goal is to study these specific catalytically driven processes.
A Wide Potential Window
The term "potential window" refers to the range of voltages an electrode can be subjected to in a given electrolyte before the electrode or solvent begins to break down.
Platinum offers a wide and useful potential window in many aqueous and non-aqueous solutions, allowing researchers to study a broad spectrum of chemical phenomena.
The Need for High Purity
The references to high-purity platinum are not trivial. Impurities on the electrode surface, even at trace levels, can act as unintended catalytic sites or block desired reactions.
Using high-purity metal is a prerequisite for obtaining clean, reliable, and reproducible electrochemical data.
Understanding the Trade-offs
No material is perfect for every situation. Acknowledging platinum's limitations is key to using it effectively.
High Cost
As a precious metal, platinum is significantly more expensive than alternative electrode materials like glassy carbon or other base metals. This can be a limiting factor for some labs or large-scale applications.
Susceptibility to "Poisoning"
Platinum's catalytically active surface can be easily "poisoned" or deactivated by contaminants.
Species like carbon monoxide, sulfides, or certain organic molecules can adsorb strongly to the surface, blocking the active sites and compromising the experiment. This necessitates rigorous cleaning and polishing protocols.
Not Universally Inert
While highly stable, platinum is not completely immune to reaction. In the presence of certain ions (like chloride) and at high positive potentials, it can slowly dissolve or form a layer of platinum oxide.
This oxide layer can, in turn, influence or interfere with the reaction being studied, a factor that must be accounted for in precise experiments.
Making the Right Choice for Your Experiment
Your choice of electrode material must be a deliberate decision based on the goals of your analysis.
- If your primary focus is general-purpose voltammetry or replicating standard methods: Platinum is often the default choice due to its well-documented behavior and wide applicability.
- If your primary focus is cost-sensitive analysis or you require a wider potential window in the positive direction: Glassy carbon is an excellent and common alternative to platinum.
- If your primary focus is studying a reaction that is undesirably catalyzed by platinum: You must select a more inert material, such as gold or glassy carbon, to obtain accurate results.
Ultimately, selecting the correct working electrode is foundational to asking a clear and answerable electrochemical question.
Summary Table:
| Property | Why It Matters for a Platinum Disk Electrode |
|---|---|
| Chemical Inertness | Resists corrosion, ensuring the measured current comes from your analyte, not the electrode. |
| Catalytic Activity | Facilitates key reactions like hydrogen evolution (HER) and oxygen reduction (ORR). |
| Wide Potential Window | Allows study of a broad spectrum of reactions before solvent/electrode breakdown. |
| Defined Surface Area | The disk geometry provides precise area for quantitative, reproducible results. |
Ready to select the perfect electrode for your specific application? The right choice is critical for obtaining clean, reliable data. KINTEK specializes in high-purity lab equipment and consumables, including a range of working electrodes for all your electrochemical needs. Let our experts help you make the right choice—contact us today to discuss your requirements and ensure your experimental success!
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Platinum Auxiliary Electrode for Laboratory Use
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Gold Disc Electrode
People Also Ask
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- What is the difference between RDE and RRDE? Unlock Advanced Electrochemical Reaction Analysis
- What is the difference between ring disk electrode and rotating disk electrode? Unlock Deeper Electrochemical Insights
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance



















