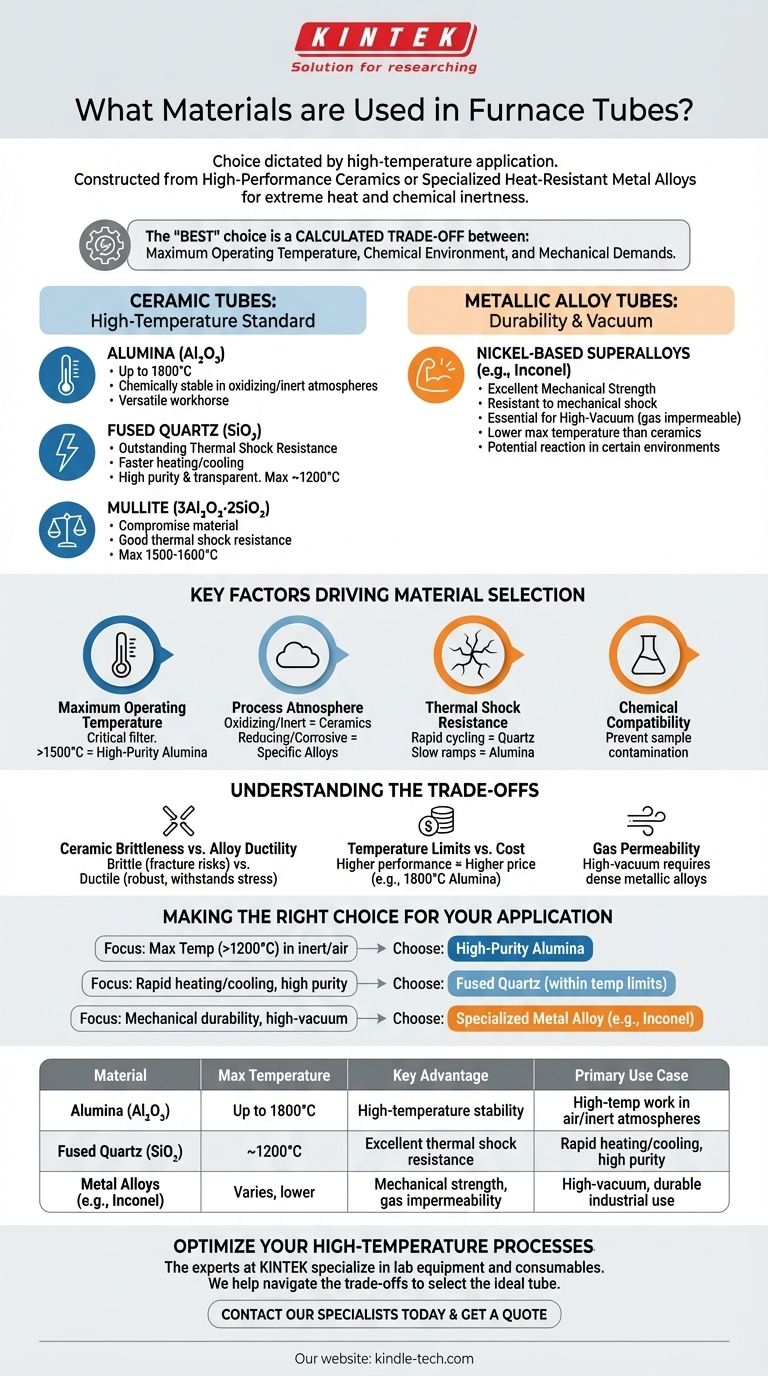
The choice of furnace tube material is dictated by the specific high-temperature application. Generally, tubes are constructed from either high-performance ceramics, such as alumina and quartz, or specialized heat-resistant metal alloys. These materials are selected to withstand extreme heat and maintain chemical inertness, ensuring the integrity of the process and the safety of the operation.
The "best" furnace tube material does not exist. The right choice is a calculated trade-off between three critical factors: the maximum required operating temperature, the chemical environment of the process, and the mechanical demands placed upon the tube.

The Two Primary Classes of Furnace Tube Materials
Furnace tubes are broadly categorized into two families: ceramics and metals. Each offers a distinct profile of strengths and weaknesses tailored to different laboratory and industrial processes.
Ceramic Tubes: The High-Temperature Standard
Ceramic tubes are favored for their exceptional heat resistance and chemical stability in most atmospheres.
Alumina (Al2O3) is the most common choice for high-temperature work, capable of operating up to 1800°C in some purities. It is chemically stable in oxidizing (air) and inert atmospheres, making it a versatile workhorse.
Fused Quartz (SiO2) is prized for its outstanding thermal shock resistance, allowing for much faster heating and cooling rates than alumina. It is also exceptionally pure and transparent, but its use is limited to temperatures below approximately 1200°C.
Mullite (3Al2O3·2SiO2) offers a compromise between alumina and quartz, providing good thermal shock resistance and a maximum use temperature around 1500-1600°C.
Metallic Alloy Tubes: For Durability and Vacuum
Metal tubes are used when mechanical strength, ductility, and impermeability to gases are more critical than sheer temperature resistance. The reference to "imported heat-resistant alloy" points to this class.
Nickel-based superalloys (like Inconel) offer excellent mechanical strength at high temperatures and are far more resistant to mechanical shock than ceramics. They are essential for high-vacuum applications where the tube must not be permeable to atmospheric gases.
These alloys, however, generally have a lower maximum operating temperature than high-purity alumina and can react in certain chemical environments, potentially contaminating the sample.
Key Factors Driving Material Selection
Choosing the correct tube is a technical decision that balances the demands of your process against the properties of the material.
Maximum Operating Temperature
This is the first and most critical filter. A process running at 1500°C immediately rules out quartz and most metallic alloys, making high-purity alumina the default choice.
Process Atmosphere
The gas inside the tube dictates material compatibility. Oxidizing (air) and inert (argon, nitrogen) atmospheres are suitable for most ceramics. Reducing atmospheres (hydrogen) or corrosive gases may require specific, and often expensive, metallic alloys.
Thermal Shock Resistance
If your process requires rapid heating or cooling cycles, the material's ability to withstand sudden temperature changes is paramount. Quartz is the clear winner in this category, while alumina requires slow, controlled temperature ramps to prevent cracking.
Chemical Compatibility
The tube material must not react with or contaminate the sample being heated. For instance, while alumina is very stable, it can react with certain materials at very high temperatures, making a non-metallic inner liner or a different tube material necessary.
Understanding the Trade-offs
Every material choice involves a compromise. Understanding these trade-offs is key to avoiding costly failures.
Ceramic Brittleness vs. Alloy Ductility
Ceramic tubes are brittle and can fracture from minor impacts or improper support. Metallic alloys are ductile and can withstand mechanical stress and vibration, making them more robust for certain industrial environments.
Temperature Limits vs. Cost
Higher performance commands a higher price. High-purity alumina capable of 1800°C is significantly more expensive than standard alumina or quartz. Exotic alloys designed for corrosive environments can also be a major cost driver.
Gas Permeability
For high-vacuum applications, gas tightness is non-negotiable. At high temperatures, ceramics can become slightly permeable to gases like helium or hydrogen. A dense metallic alloy tube is often the only reliable solution for maintaining a hard vacuum.
Making the Right Choice for Your Application
Selecting the right material ensures the accuracy, repeatability, and safety of your work. Use your primary goal as the starting point for your decision.
- If your primary focus is maximum temperature (>1200°C) in inert or air atmospheres: High-purity Alumina (Al2O3) is the industry standard.
- If your primary focus is rapid heating/cooling cycles and high sample purity: Fused Quartz is the ideal choice, provided you stay within its temperature limits.
- If your primary focus is mechanical durability or high-vacuum integrity: A specialized heat-resistant metal alloy, such as Inconel, is necessary.
By aligning your material choice with your specific temperature, atmosphere, and mechanical needs, you ensure the safety and success of your high-temperature process.
Summary Table:
| Material | Max Temperature | Key Advantage | Primary Use Case |
|---|---|---|---|
| Alumina (Al₂O₃) | Up to 1800°C | High-temperature stability | High-temp work in air/inert atmospheres |
| Fused Quartz (SiO₂) | ~1200°C | Excellent thermal shock resistance | Rapid heating/cooling, high purity |
| Metal Alloys (e.g., Inconel) | Varies, lower than ceramics | Mechanical strength, gas impermeability | High-vacuum, durable industrial use |
Optimize your high-temperature processes with the right furnace tube.
Choosing the correct tube material is critical for the safety, efficiency, and success of your work. The experts at KINTEK specialize in lab equipment and consumables. We can help you navigate the trade-offs between temperature, atmosphere, and mechanical requirements to select the ideal tube for your specific application—whether you need the extreme heat resistance of alumina, the rapid cycling of quartz, or the vacuum integrity of a metal alloy.
Contact our specialists today to discuss your needs and ensure you get the right solution for your laboratory.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory High Pressure Vacuum Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the test for refractory material? Essential Tests for High-Temperature Performance
- Why are alumina and graphite powder used as auxiliary consumables in tube furnace heat treatment? Prevent Degradation
- What is the temperature of a quartz tube? Understanding the 1200°C Limit and Its Critical Conditions
- Why is it necessary to use high-alumina support racks in high-temperature corrosion experiments? Ensure Data Accuracy
- Why are zirconia grinding jars and balls required for halide electrolytes? Ensure Pure ZrO2-Li2ZrCl6 Synthesis
- What is the purpose of using a stirring device with an ice bath? Master Cellulose/Ag2S Composite Synthesis
- How do oil-free vacuum pumps differ from oil-sealed vacuum pumps in terms of operation? A Guide to Performance vs. Purity
- Why is high-temperature sealant used to seal alumina crucibles? Achieve Flawless Alumina Coating Results



















