The concept of a single melting temperature is a misconception in advanced manufacturing. While a pure material like ice has a fixed melting point, the 600°C you're asking about refers to a specific step in a complex process like metal injection molding (MIM) or 3D printing. This temperature is for debinding—burning away a sacrificial polymer binder—not for melting the actual metal, which occurs at a much higher temperature.
The critical takeaway is that modern fabrication processes use a sequence of carefully controlled temperatures. Confusing a debinding temperature with a melting point can lead to catastrophic failure, as one process is designed to create a porous structure while the other creates a fully dense, solid object.

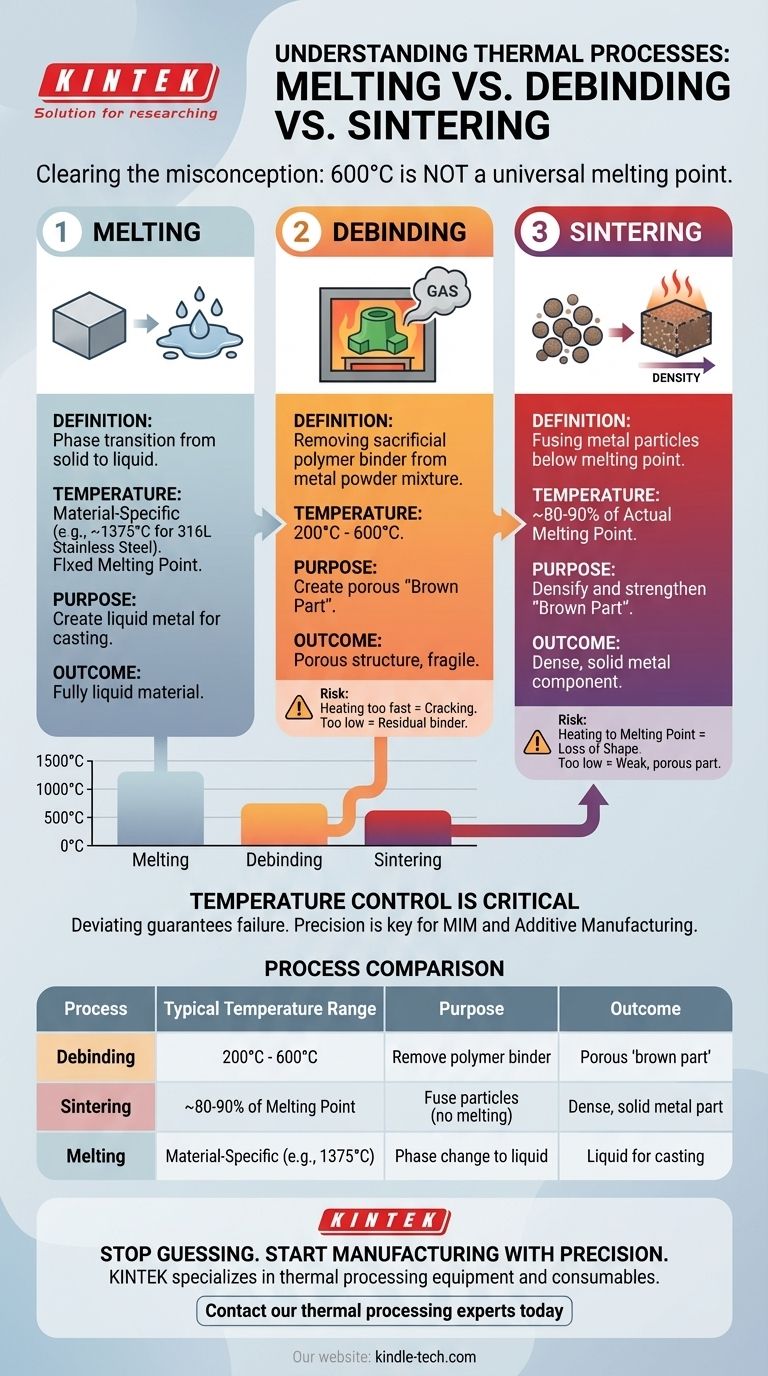
The Difference: Melting, Debinding, and Sintering
To understand why 600°C is not a universal melting point, we must distinguish between three distinct thermal processes. Each has a fundamentally different purpose.
What is Melting?
Melting is the phase transition of a substance from a solid to a liquid. This occurs at a specific temperature known as the melting point, which is a fundamental property of a material.
For example, water melts at 0°C (32°F), while 316L stainless steel, a common material in MIM, melts at approximately 1375°C (2500°F).
What is Debinding?
Debinding is an intermediate step used in processes that fabricate parts from a mixture of metal powder and a polymer binder. The initial part, known as a "green part," is solid but fragile.
This part is heated in a furnace, typically to a range of 200°C to 600°C. The goal is to slowly burn away the binder material, leaving behind a porous structure of metal powder. The 600°C figure represents the upper end of this process, ensuring all binder is removed without affecting the metal particles.
What is Sintering?
After debinding, the fragile, porous part (now called a "brown part") undergoes sintering. It is heated to a much higher temperature, but one that is just below the metal's actual melting point.
At this high temperature, atomic diffusion occurs at the contact points between the metal powder particles. They fuse together, causing the part to shrink and densify into a solid, strong metal component. The part never becomes a liquid.
Understanding the Trade-offs of Temperature Control
Precise temperature control is the most critical factor in these processes. Deviating from the ideal thermal profile guarantees failure, but the reasons for failure are different at each stage.
The Risk of Heating Too Low
If the debinding temperature is too low, the binder will not be fully removed. This residual binder becomes a contaminant during sintering, leading to a weak, brittle, or flawed final part.
If the sintering temperature is too low, the metal particles will not fuse adequately. The resulting part will be overly porous and lack the required mechanical strength and density.
The Risk of Heating Too High
Heating too rapidly during debinding can cause the outgassing binder to build up pressure and crack the part. This is why it's a slow, controlled ramp-up.
Heating the part to its actual melting point during the sintering phase is the ultimate failure. The part would lose its shape, slump under its own weight, and become a useless puddle of metal. Sintering relies on maintaining the part's geometry right up to the edge of melting.
Making the Right Choice for Your Goal
Understanding the intent behind a thermal process is more important than memorizing a specific number. The temperature's purpose dictates the entire operation.
- If your primary focus is to create a solid metal part from powder: You must use a multi-stage process. First, debind at a lower temperature (e.g., up to 600°C) to remove the binder, then sinter at a much higher temperature just below the metal's true melting point.
- If your primary focus is to simply cast a metal: You only need to know that material's specific melting point and heat it until it becomes fully liquid before pouring it into a mold.
- If your primary focus is to interpret a technical specification: Never assume a temperature is a melting point. It is far more likely to be a debinding, curing, or annealing temperature, each of which serves a unique and non-destructive purpose.
Ultimately, temperature is a tool, and knowing the difference between melting, debinding, and sintering is what separates successful fabrication from costly failure.
Summary Table:
| Process | Typical Temperature Range | Purpose | Outcome |
|---|---|---|---|
| Debinding | 200°C - 600°C | Remove polymer binder from metal powder | Porous 'brown part' |
| Sintering | ~80-90% of Melting Point | Fuse metal particles without melting | Dense, solid metal part |
| Melting | Material-Specific (e.g., 1375°C for 316L Steel) | Phase change from solid to liquid | Liquid metal for casting |
Stop guessing temperatures and start manufacturing with precision.
Confusing debinding with melting can ruin parts and waste resources. KINTEK specializes in the precise thermal processing equipment and consumables your laboratory needs for successful metal injection molding (MIM) and additive manufacturing.
We provide the reliable furnaces and expert support to ensure your debinding and sintering profiles are perfect every time, preventing costly failures and delivering strong, dense metal parts.
Contact our thermal processing experts today to discuss your application and ensure your next project is a success.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the difference between oven incubator and muffle furnace? Choose the Right Lab Heating Tool
- How do you use a muffle furnace in a lab? A Step-by-Step Guide to Safe & Precise Operation
- What are the uses of muffle furnaces? Achieve Precise, Contamination-Free High-Temperature Processing
- How do you check the temperature of a muffle furnace? A Guide to Precise Monitoring
- What is the use of muffle furnace in pharmaceuticals? Essential for Purity & Quality Control



















