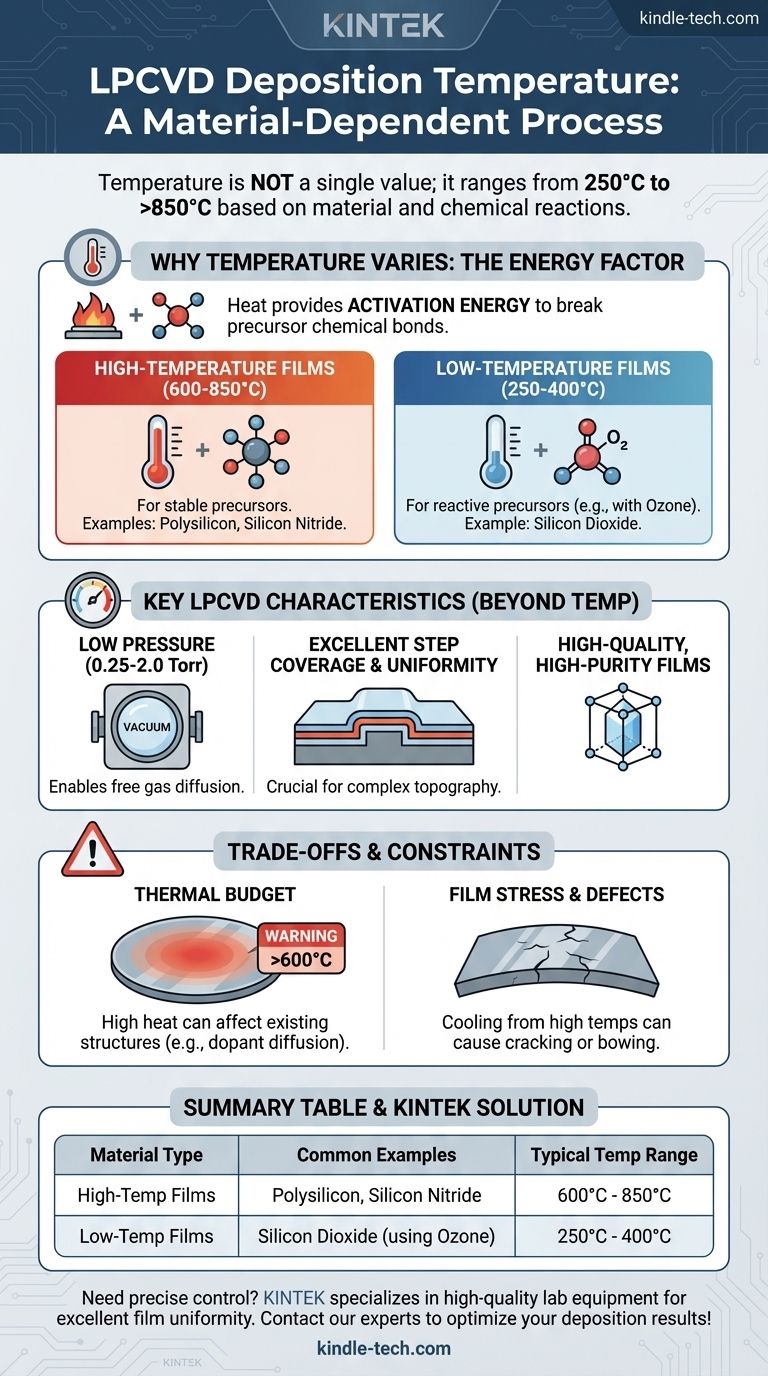
The deposition temperature for Low-Pressure Chemical Vapor Deposition (LPCVD) is not a single value; it is highly dependent on the specific material being deposited. LPCVD temperatures typically range from as low as 250°C for certain oxides to over 850°C for materials like polysilicon. This wide operating window is a direct result of the different chemical reactions required to form each film.
The critical factor determining LPCVD temperature is the activation energy needed for the specific chemical reaction. High-quality films like polysilicon require significant thermal energy to break down stable precursor gases, whereas catalyzed reactions for films like silicon dioxide can proceed at much lower temperatures.

Why Temperature Varies by Material
The temperature of an LPCVD process is fundamentally about providing enough energy to initiate and sustain the desired chemical reaction on the substrate surface. Different materials are formed from different precursors, each with its own energy requirement.
The Principle of Thermal Energy
In LPCVD, heat is the primary catalyst. It provides the activation energy needed to break the chemical bonds of the reactant gases, allowing atoms to deposit and form a solid film on the wafer.
High-Temperature Films (600-850°C)
Films that require the decomposition of very stable molecules demand high temperatures.
Polysilicon and silicon nitride are prime examples. These processes often use precursors like silane (SiH₄) and dichlorosilane (SiH₂Cl₂), which require temperatures in the 600°C to 850°C range to break down efficiently and form a dense, uniform film.
Low-Temperature Films (250-400°C)
Some LPCVD processes can run at significantly lower temperatures by using more reactive precursors or co-reactants that lower the required activation energy.
A common example is the deposition of silicon dioxide (SiO₂) using ozone (O₃). The high reactivity of ozone allows the process to run effectively at temperatures between 250°C and 400°C, which is much lower than other thermal oxide depositions.
Key Characteristics of the LPCVD Process
Beyond temperature, the defining feature of LPCVD is its operating pressure, which directly influences the quality of the deposited film.
The Role of Low Pressure
By operating at very low pressures (0.25 to 2.0 Torr), the movement of gas molecules becomes less obstructed. This allows the reactant gases to diffuse more freely and evenly across all wafer surfaces.
This low-pressure environment is the reason LPCVD provides excellent step coverage and film uniformity, even over complex topography. Unlike higher-pressure methods, it does not require a carrier gas.
Excellent Film Quality
The controlled, thermally-driven nature of the process gives engineers precise control over the film's structure and composition. This results in high-purity films with reliable and repeatable properties, crucial for the semiconductor industry.
Understanding the Trade-offs
While powerful, the temperatures required for LPCVD create important constraints that engineers must manage.
Thermal Budget Constraints
The primary trade-off of high-temperature LPCVD is the thermal budget. Exposing a wafer to high temperatures (above 600°C) can affect previously fabricated structures on the device.
For example, high heat can cause dopants to diffuse out of their intended regions, potentially altering the electrical performance of transistors. This is why lower-temperature deposition methods are often required in later manufacturing stages.
Film Stress and Defects
Depositing films at high temperatures can induce significant mechanical stress as the wafer cools down. This stress can lead to film cracking or cause the entire wafer to bow, creating problems for subsequent lithography steps.
Making the Right Choice for Your Process
Your choice of deposition temperature is dictated by the material needed and its integration into the overall device fabrication flow.
- If your primary focus is creating a gate contact or structural layer: You will almost certainly use a high-temperature (600°C+) process to deposit high-quality polysilicon.
- If your primary focus is depositing a dielectric over temperature-sensitive components: You should utilize a lower-temperature (250-400°C) LPCVD process, such as an ozone-based silicon dioxide deposition.
- If your primary focus is achieving the best possible conformal coating on a complex surface: The low-pressure nature of LPCVD is its key advantage, making it superior to many other CVD techniques regardless of the specific temperature.
Ultimately, understanding the relationship between the material, the required reaction energy, and the process temperature is key to successfully leveraging LPCVD.
Summary Table:
| Material Type | Common Examples | Typical LPCVD Temperature Range |
|---|---|---|
| High-Temperature Films | Polysilicon, Silicon Nitride | 600°C - 850°C |
| Low-Temperature Films | Silicon Dioxide (using Ozone) | 250°C - 400°C |
Need precise temperature control for your LPCVD processes? KINTEK specializes in high-quality lab equipment and consumables for semiconductor fabrication. Our expertise ensures you achieve excellent film uniformity and step coverage for materials like polysilicon and silicon dioxide. Contact our experts today to optimize your deposition results!
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vertical Laboratory Tube Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
People Also Ask
- What is a CVD tube furnace? A Complete Guide to Thin-Film Deposition
- What function does CVD equipment serve in rhodium-modified coatings? Achieve Deep Diffusion and Microstructural Precision
- How does chirality affect carbon nanotubes? It Determines If They Are Metal or Semiconductor
- What is the floating catalyst method? A Guide to High-Yield CNT Production
- What are the main advantages of Chemical Vapor Deposition (CVD)? Achieve Precision Coating for Complex Geometries



















