In short, crucibles have been used continuously from the dawn of metallurgy in the 5th and 6th millennia BCE through to the present day. Their history is not a single period but a constant evolution, mirroring humanity's growing mastery over high temperatures for processing metals, glass, and other advanced materials. They are as fundamental to a modern semiconductor lab as they were to a Bronze Age metalworker.
The crucible is more than just a high-temperature bowl; it is a foundational technology. Its form and material at any given point in history serve as a direct indicator of the technological capabilities and ambitions of that era.

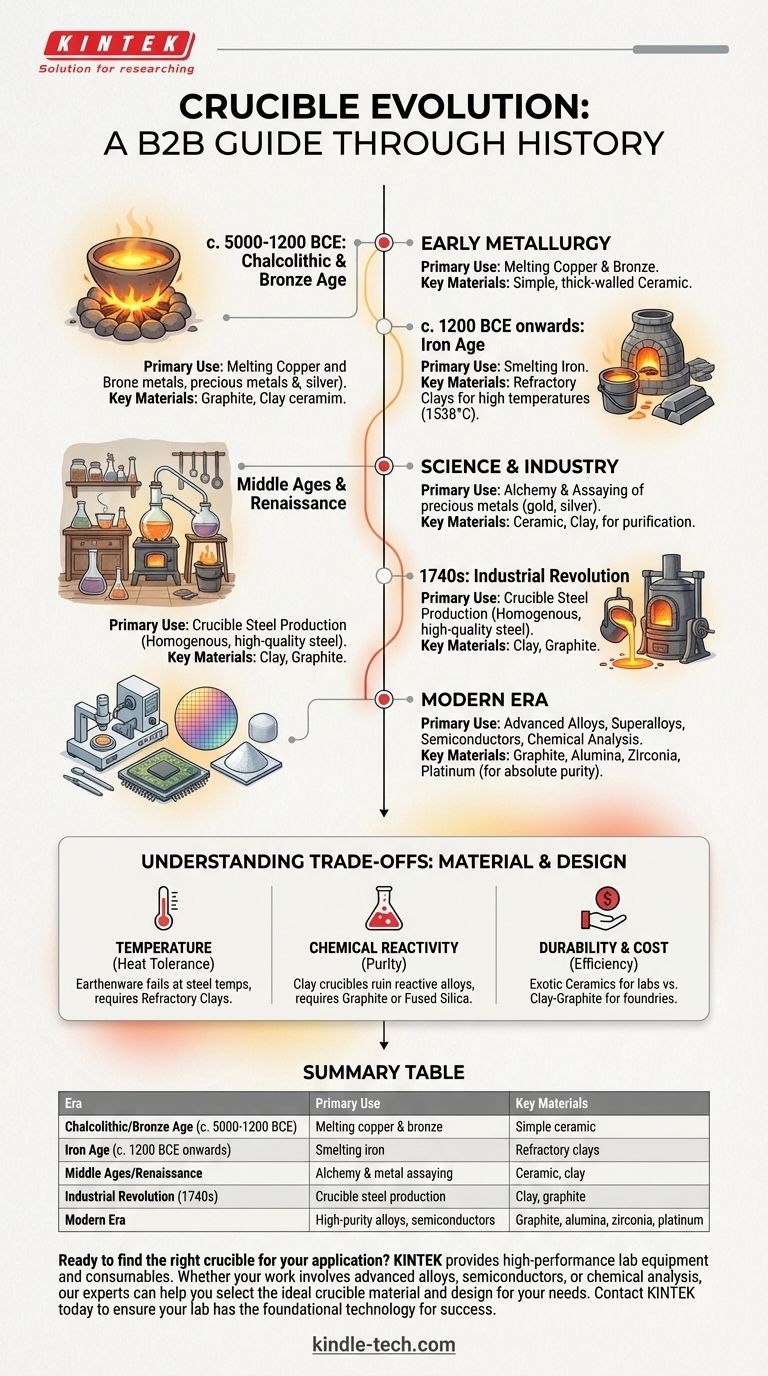
The Origins: Early Metallurgy
The earliest uses of the crucible are tied directly to humanity's first experiments with metal. It was the essential tool that allowed for the transition from using native metals found on the surface to extracting metals from ore.
The Chalcolithic and Bronze Age (c. 5000-1200 BCE)
The first crucibles were simple, thick-walled ceramic bowls. Archaeologists have found examples dating back to the Chalcolithic (Copper Age) in the Near East and Eastern Europe.
These early crucibles were used for melting copper and later for creating bronze, an alloy of copper and tin. Their primary function was to contain the molten metal after it was smelted from ore, allowing it to be poured into molds for tools, weapons, and ornaments.
The Iron Age (c. 1200 BCE onwards)
Working with iron required significantly higher temperatures (around 1538°C or 2800°F) than copper or bronze. This technical challenge drove innovation in both furnace and crucible technology.
Crucibles from this period needed to be made from more refractory clays capable of withstanding the intense heat without cracking. The design also began to evolve, sometimes including lids to help control the atmosphere inside and prevent impurities from contaminating the metal.
The Crucible in Science and Industry
As societies became more complex, the crucible's role expanded from simple metal casting to a tool for precise chemical analysis and industrial-scale production.
Alchemy and Early Assaying
From the Hellenistic period through the Middle Ages and into the Renaissance, the crucible was the central apparatus of the alchemist. It was used in attempts to transmute base metals into gold and in the distillation and purification of substances.
More practically, crucibles were indispensable for assaying—the process of determining the content and purity of precious metals like gold and silver. This was a critical function for trade, taxation, and coinage, making the crucible a key tool of economic control.
The Industrial Revolution and Crucible Steel
A pivotal moment in the crucible's history came in the 1740s when Benjamin Huntsman, a clockmaker in England, invented the crucible steel process. By melting blister steel and other ingredients in a sealed clay crucible, he was able to produce a homogenous, high-quality steel for the first time.
This innovation was a catalyst for the Industrial Revolution, providing the superior metal needed for more precise tools, durable machine parts, and stronger springs.
Understanding the Trade-offs: Material and Design
The story of the crucible is a story of engineering trade-offs. The "best" crucible has always been defined by the specific task it needed to perform.
The Constraint of Temperature
The single biggest limiting factor has always been heat tolerance. A simple earthenware crucible that works for lead or tin will fail catastrophically at the temperatures needed for steel or platinum. The evolution from clay to graphite, alumina, and zirconia is a direct response to the need to melt ever more demanding materials.
The Problem of Chemical Reactivity
A crucible must not only withstand the heat but also resist chemical reaction with the molten material inside it. Melting a highly reactive alloy in a simple clay crucible can introduce silicon and aluminum impurities, ruining the final product.
This is why modern applications use specific crucible materials: graphite for non-ferrous metals, fused silica for high-purity silicon, and even platinum for specialty glass manufacturing to ensure absolute purity.
Balancing Durability and Cost
A highly durable, multi-use crucible made of an exotic ceramic is ideal for a laboratory setting but may be too expensive for a large foundry. In industrial casting, cheaper, often single-use clay-graphite or silicon carbide crucibles provide the necessary performance at an acceptable cost.
How to Apply This to Your Goal
The historical significance of the crucible depends entirely on the lens through which you view it.
- If your primary focus is ancient history and archaeology: View the crucible as a key diagnostic artifact that reveals a culture's level of metallurgical sophistication and trade networks.
- If your primary focus is the history of science: See the crucible as the essential laboratory vessel that enabled the shift from mystical alchemy to quantitative chemistry.
- If your primary focus is industrial and materials engineering: Recognize the crucible as a foundational technology whose material evolution was a prerequisite for creating the advanced alloys, superalloys, and semiconductors that define our modern world.
Ultimately, the crucible's continuous presence throughout history demonstrates a fundamental human drive: to control fire and transform materials.
Summary Table:
| Era | Primary Use | Key Materials |
|---|---|---|
| Chalcolithic/Bronze Age (c. 5000-1200 BCE) | Melting copper & bronze | Simple ceramic |
| Iron Age (c. 1200 BCE onwards) | Smelting iron | Refractory clays |
| Middle Ages/Renaissance | Alchemy & metal assaying | Ceramic, clay |
| Industrial Revolution (1740s) | Crucible steel production | Clay, graphite |
| Modern Era | High-purity alloys, semiconductors | Graphite, alumina, zirconia, platinum |
Ready to find the right crucible for your application? The history of the crucible is one of continuous innovation to meet specific thermal and chemical challenges. KINTEK specializes in providing high-performance lab equipment and consumables, including crucibles for a wide range of materials and temperatures. Whether your work involves advanced alloys, semiconductors, or chemical analysis, our experts can help you select the ideal crucible material and design for your needs. Contact KINTEK today to ensure your lab has the foundational technology for success.
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- What is the purpose of using an alumina crucible with mother powder for Ga-LLZO? Ensure Pure Sintering Results
- How does a crucible furnace work? A Guide to Melting Metals Efficiently
- What temperature is an Al2O3 crucible? Key Factors for High-Temperature Success Up to 1700°C
- What role does a nickel crucible play during the alkali fusion? Ensure Safe & Efficient Zeolite Synthesis
- What are the primary functions of alumina crucibles for calcining LLZO? Optimize Your Solid Electrolyte Synthesis
- What are the functional advantages of using high-purity alumina crucibles? Achieve Precise Oxidation Data
- What are the advantages of using a nickel crucible? Ensure Safety and Purity in Lithium Smelting
- What are the advantages of using high-purity quartz crucibles? Ensure Purity in Fe-Co Alloy Melt-Spinning



















