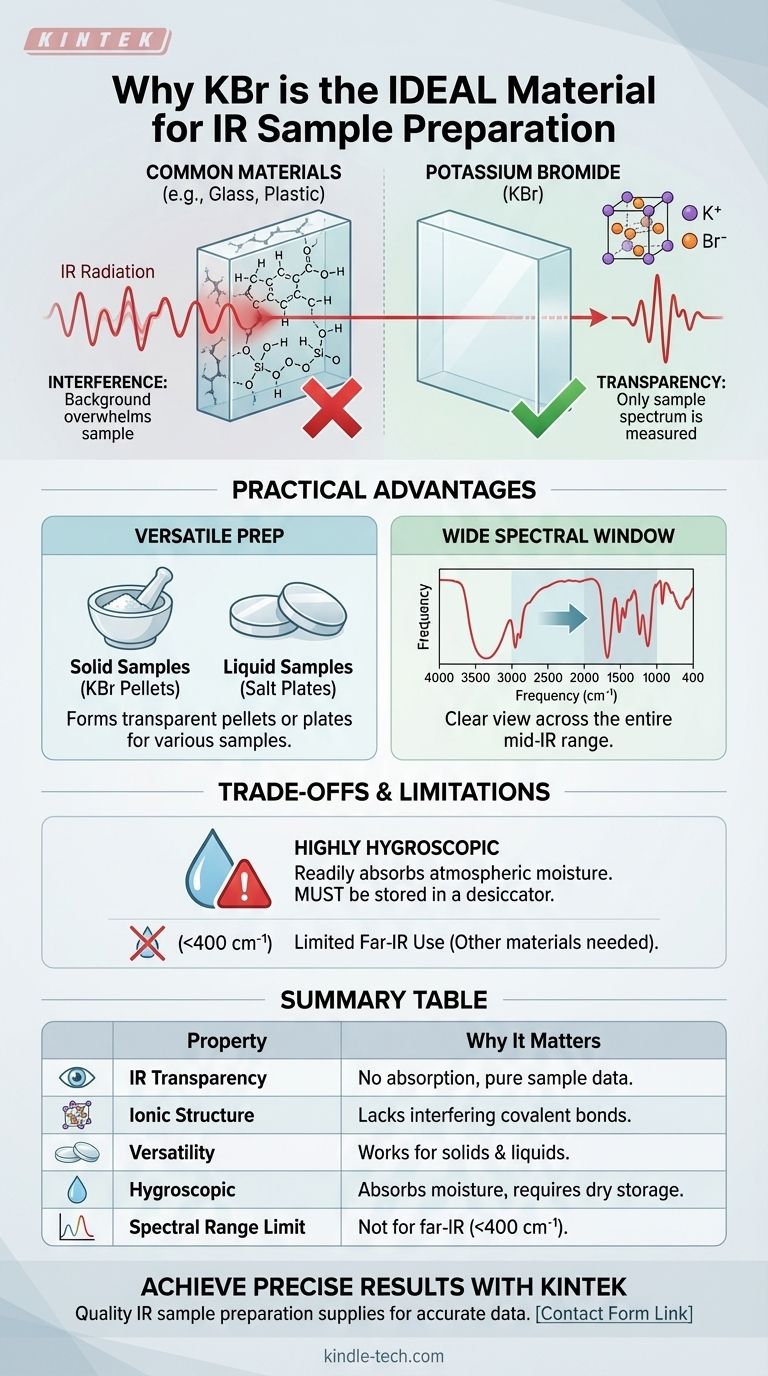
In short, Potassium Bromide (KBr) is an ideal material for most infrared (IR) spectroscopy sample preparations because it is transparent to IR radiation. This means it does not absorb light in the mid-infrared range, ensuring that the spectrum you measure belongs only to your sample and is not obscured by the material holding it.
The central challenge in IR spectroscopy is isolating the sample's spectrum from its surroundings. KBr's simple ionic structure lacks the types of chemical bonds that absorb mid-range IR light, making it the perfect "invisible" window or matrix for analyzing most organic and inorganic compounds.

The Fundamental Principle: Infrared Transparency
To understand why KBr is so effective, we must first understand what the IR spectrometer "sees." The instrument measures how a sample's chemical bonds vibrate and stretch when they absorb infrared light at specific frequencies.
What "Transparent" Means in IR Spectroscopy
For a material to be useful as a sample holder, it must not absorb IR light in the same region as the sample. This property is called infrared transparency.
Just as clear glass is transparent to visible light, allowing us to see through it, KBr is transparent to the mid-infrared light (typically 4000 cm⁻¹ to 400 cm⁻¹) used for analysis.
The Problem with Most Materials
Common materials like glass, quartz, and plastics are made of molecules with covalent bonds (e.g., Si-O, C-C, C-H). These bonds are the very things that absorb IR radiation.
Using a sample holder made of these materials would be like trying to take a picture through a lens that has its own picture printed on it—the background would completely overwhelm the subject.
Why Ionic Salts Are Different
KBr is an ionic salt. It consists of a crystal lattice of potassium cations (K⁺) and bromide anions (Br⁻).
The vibrations of this simple ionic lattice occur at very low frequencies, far below the 400 cm⁻¹ cutoff for standard mid-IR analysis. As a result, KBr has no absorption bands of its own in the region of interest, giving you a clean, unobstructed baseline.
Practical Advantages of Using KBr
Beyond its IR transparency, KBr has several physical properties that make it highly practical for laboratory use.
Wide Spectral Window
KBr offers a clear and unobstructed view across the entire mid-IR range. This allows for the analysis of a vast array of chemical bonds, from O-H stretches at high frequencies to complex "fingerprint" region vibrations at lower frequencies.
Versatility in Sample Preparation
KBr's properties allow it to be used for both liquid and solid samples, making it a highly versatile tool.
- For Liquid Samples (Salt Plates): KBr can be polished into flat, transparent discs called "salt plates." A drop of a liquid sample is sandwiched between two plates, creating a thin film of uniform thickness that is ready for analysis.
- For Solid Samples (KBr Pellets): A solid sample can be finely ground with pure, dry KBr powder. This mixture is then put under immense pressure to form a small, transparent pellet. The KBr acts as a matrix, holding the sample particles in place for the IR beam to pass through.
Understanding the Trade-offs and Limitations
While KBr is the standard for most applications, it is not without its weaknesses. A true expert understands the limitations of their tools.
It is Highly Hygroscopic
The most significant drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
Water (H₂O) has very strong, broad IR absorption bands. If your KBr plates or powder absorb water, these bands will appear in your spectrum, potentially obscuring your sample's data. This is why KBr must always be stored in a desiccator and handled efficiently.
Limited Use in the Far-IR Region
The transparency of KBr ends around 400 cm⁻¹. For analysis in the far-infrared region, which is used to study heavy atom vibrations and crystal lattice modes, other materials like Cesium Iodide (CsI) or specialized polyethylene windows are required.
Potential for Sample Reactivity
Although rare, KBr can react with certain types of samples. For example, some complex halide salts can undergo ion exchange with the bromide in the KBr matrix, altering the sample and leading to an inaccurate spectrum.
Making the Right Choice for Your Goal
Understanding the properties of KBr allows you to prepare samples correctly and interpret your results with confidence.
- If your primary focus is analyzing a liquid or solution: Use a pair of clean, dry KBr salt plates to create a thin film of your sample.
- If your primary focus is analyzing a solid: Grind your sample thoroughly with dry KBr powder and press it into a uniform, transparent pellet.
- If you see broad, unexpected peaks around 3400 cm⁻¹ and 1640 cm⁻¹: Your KBr is likely contaminated with water, and the plates or powder should be dried or replaced.
Ultimately, mastering IR spectroscopy begins with understanding why a simple salt is the key to revealing the complex world of molecular vibrations.
Summary Table:
| Property | Why It Matters for IR Spectroscopy |
|---|---|
| IR Transparency | Does not absorb mid-IR light (4000-400 cm⁻¹), ensuring only sample spectrum is measured. |
| Ionic Structure | Lacks covalent bonds that absorb IR, providing a clean baseline. |
| Versatility | Can be used for both solid (KBr pellets) and liquid (salt plates) samples. |
| Hygroscopic Nature | Absorbs moisture, which can contaminate spectra; requires dry storage. |
| Spectral Range Limit | Not suitable for far-IR (<400 cm⁻¹); alternatives like CsI are needed. |
Achieve precise and reliable IR spectroscopy results with the right lab equipment. KINTEK specializes in providing high-quality IR sample preparation supplies, including KBr pellets and plates, designed for accuracy and ease of use. Whether you're analyzing organic compounds, pharmaceuticals, or inorganic materials, our products help you avoid common pitfalls like moisture contamination and ensure clear spectral data. Contact us today to explore our range of spectroscopy consumables and elevate your laboratory's capabilities!
Visual Guide
Related Products
- kbr pellet press 2t
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
People Also Ask
- Why only KBr is used in IR spectroscopy? The Truth About the Best Material for Your Sample
- Why do we use KBr in IR spectroscopy? Achieve Clear, High-Quality Solid Sample Analysis
- How do you prepare a KBr pellet for IR spectroscopy? Master the Key Steps for a Clear Spectrum
- What are the safety precautions for KBr? Achieve Flawless FTIR Pellet Preparation and Data Accuracy
- Why KBr is used for IR spectroscopy? The Ideal Medium for Solid Sample Analysis



















