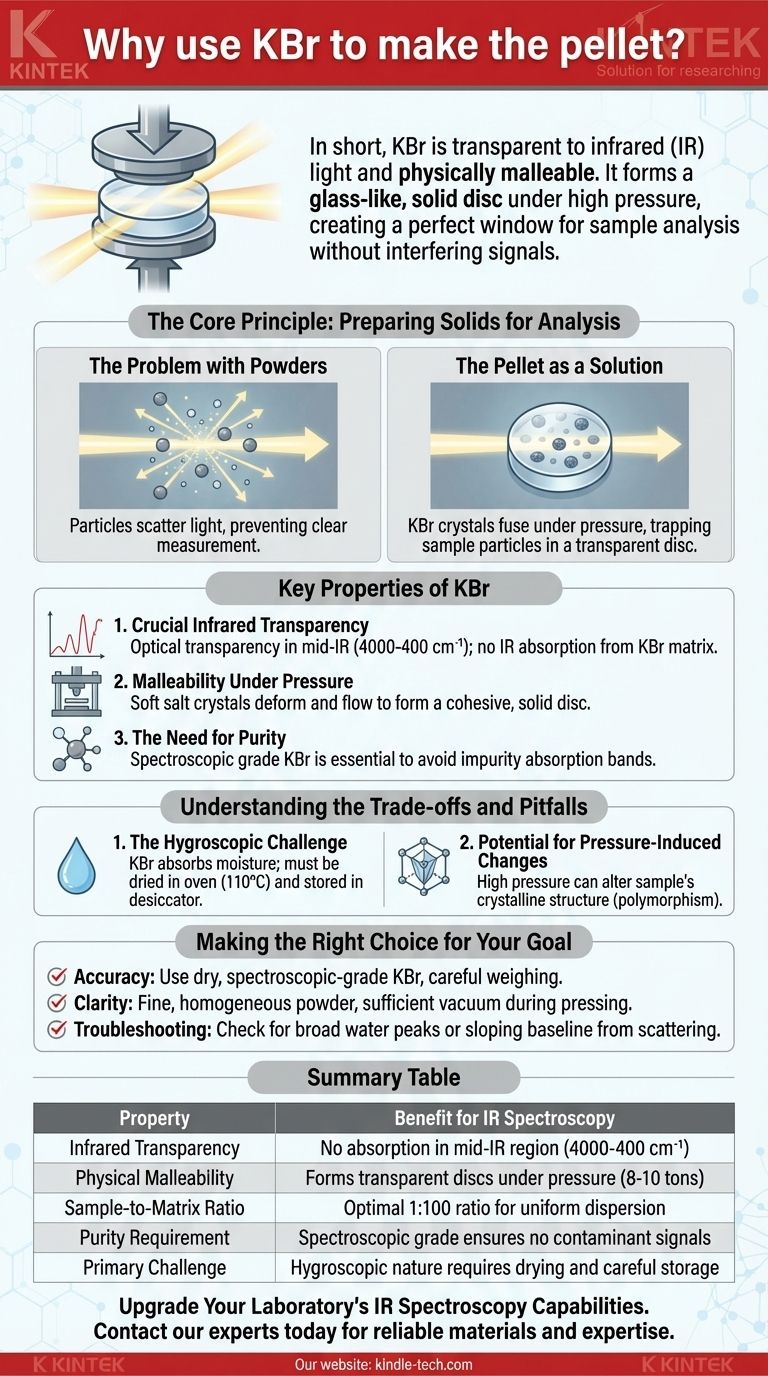
In short, KBr (Potassium Bromide) is used to make pellets for infrared (IR) spectroscopy because it is transparent to infrared light and physically malleable. Its crystalline structure allows it to become a glass-like, solid disc under high pressure, creating a perfect window to hold the sample for analysis without producing an interfering signal of its own.
The core challenge in analyzing solid samples with IR spectroscopy is getting light to pass through them uniformly. KBr serves as an ideal matrix because it solves this problem both optically (it's invisible to the IR beam) and physically (it forms a transparent solid pellet).

The Core Principle: Preparing Solids for Analysis
The Problem with Powders
To get an accurate infrared spectrum, the IR beam must pass through your sample. Simply placing a powdered solid in the beam path doesn't work effectively because the particles scatter the light in all directions, preventing a clear measurement.
The goal is to suspend the sample particles in a solid matrix that is completely transparent to infrared radiation. This allows the beam to pass through and interact only with the sample material.
The Pellet as a Solution
The KBr pellet method accomplishes this by mixing a tiny amount of the sample (typically a 1:100 ratio) with a large amount of KBr powder. When pressed under immense pressure (8-10 tons), the soft KBr salt crystals fuse together into a semi-transparent or transparent disc, trapping the sample particles within it.
Key Properties of KBr
Crucial Infrared Transparency
The single most important property of KBr is its optical transparency across the entire mid-infrared region (4000-400 cm⁻¹). This means the KBr matrix itself does not absorb the IR light, so any absorption recorded in the spectrum comes purely from your sample, not the matrix holding it.
Malleability Under Pressure
KBr is a soft alkali halide salt. Unlike harder materials that would shatter, KBr has the ability to deform and flow under high pressure. This property allows the fine powder to fuse into a cohesive, solid disc without melting, creating a high-quality optical window.
The Need for Purity and Consistency
The quality of your spectrum is directly dependent on the quality of your KBr. Using spectroscopic grade KBr is essential because any impurities will have their own IR absorption bands, which will appear in your spectrum and contaminate your results.
Achieving a Homogeneous Mixture
The references to a 100:1 KBr-to-sample ratio and the need for thorough grinding are about creating a uniform dispersion. If the sample isn't mixed properly, clumps will cause light scattering and result in an inaccurate spectrum with a sloping baseline.
Understanding the Trade-offs and Pitfalls
The Hygroscopic Challenge
The primary drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong and broad IR absorption peaks that can easily overwhelm the signals from your actual sample.
This is why KBr powder must be dried in an oven (around 110°C) before use and stored in a desiccator. It is also why background scans are run with a pure KBr pellet to help subtract the signal from any residual moisture.
Potential for Pressure-Induced Changes
The high pressure used to form the pellet can, in some cases, alter the crystalline structure of the sample itself. This phenomenon, known as polymorphism, can lead to slight changes in the resulting spectrum.
The Risk of Poor Pellet Quality
A cloudy or fractured pellet indicates a problem. This is often caused by insufficient grinding, inadequate vacuum during pressing (which traps air), or the KBr being too moist. A poor-quality pellet will scatter light excessively, leading to noisy and unreliable data.
Making the Right Choice for Your Goal
To obtain an accurate spectrum, you must treat the preparation of the KBr pellet as a critical part of the experiment itself.
- If your primary focus is accuracy: Always use dry, spectroscopic-grade KBr and carefully weigh your materials to maintain the correct sample-to-KBr ratio.
- If your primary focus is clarity and low noise: Ensure the sample and KBr are ground together into an extremely fine, homogenous powder and apply sufficient vacuum during pressing.
- If you are troubleshooting a poor spectrum: First, look for the broad, characteristic peaks of water. Second, check for a sloping baseline, which indicates light scattering from a poorly mixed or pressed pellet.
Ultimately, understanding why KBr is chosen—for its unique combination of optical transparency and physical malleability—empowers you to create better samples and generate more reliable data.
Summary Table:
| Property | Benefit for IR Spectroscopy |
|---|---|
| Infrared Transparency | No absorption in mid-IR region (4000-400 cm⁻¹) |
| Physical Malleability | Forms transparent discs under pressure (8-10 tons) |
| Sample-to-Matrix Ratio | Optimal 1:100 ratio for uniform dispersion |
| Purity Requirement | Spectroscopic grade ensures no contaminant signals |
| Primary Challenge | Hygroscopic nature requires drying and careful storage |
Upgrade Your Laboratory's IR Spectroscopy Capabilities
Struggling with inconsistent results or noisy spectra from your solid sample analysis? The quality of your KBr pellets directly impacts your data accuracy.
KINTEK specializes in high-purity, spectroscopic-grade lab equipment and consumables designed specifically for reliable IR spectroscopy. We provide the materials and expertise to help you:
- Achieve crystal-clear spectra with minimal baseline noise
- Eliminate water contamination through proper drying and storage solutions
- Optimize your sample preparation technique for reproducible results
Don't let pellet preparation challenges compromise your research. Contact our experts today to discuss how KINTEK's laboratory solutions can enhance your spectroscopy workflow and deliver the reliable data you need.
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- How do you prepare a KBr pellet for IR spectroscopy? Master the Key Steps for a Clear Spectrum
- How do you prepare samples for infrared spectroscopy? Master Solid, Liquid & Gas Techniques
- What is KBr disc method in IR spectroscopy? A Guide to Solid Sample Analysis
- What are the safety precautions for KBr? Achieve Flawless FTIR Pellet Preparation and Data Accuracy
- Why only KBr is used in IR spectroscopy? The Truth About the Best Material for Your Sample



















